Abstract
Pfaffia glomerata, popularly known as Brazilian-ginseng, stands out as a species of medicinal interest that has a high photoautotrophic potential for in vitro cultivation. This study aimed to analyze cell wall components of P. glomerata during in vitro cultivation in a CO2-enriched atmosphere. For this, P. glomerata plants were grown in MS medium without sucrose, in acrylic chambers with continuous forced air ventilation at 400 and 1000 μL L−1 CO2, and a control treatment with flasks put outside the chambers, without forced ventilation. The experiment was evaluated at 20, 30 and 40 days of cultivation, totaling nine treatments in a 3 × 3 factorial scheme (CO2 concentration × days), with 4 replications. Analyses of growth, photosynthesis and cell wall immunohistochemistry (using monoclonal antibodies JIM7, JIM13 and LM10) were done. The CO2 enrichment at the concentration of 1000 μL L−1 induced greater growth and accumulation of dry mass, in addition to increasing the photosynthetic rate. Immunohistochemistry analyses showed that the presence of homogalacturonan pectins detected by the JIM7 antibody decreased from 20 to 40 days, regardless of CO2 treatment. The deposition of heteroxylan and the JIM13 AGP epitope was detected exclusively in the secondary wall regions, with higher intensity in the treatment of 1000 µL L−1 CO2. This work opens new perspectives to understanding the dynamics between photoautotrophy and cell wall deposition in P. glomerata.
Key message
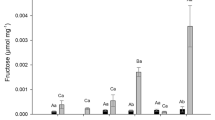
P. glomerata under elevated CO2 produces more pectic polysaccharides and hemicelluloses in the cell wall; increasing pectic polymers and xylans are associated with dry biomass gain in P. glomerata under high CO2.



Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Avci U, Pattathil S, Hahn MG (2012) Immunological approaches to plant cell wall and biomass characterization: immunolocalization of glycan epitopes. In: Himmel ME (ed) Biomass conversion: methods and protocols. Humana Press, New York, p 73
Ávila RT, Almeida WL, Costa LC, Machado KL, Barbosa ML, Souza RPB, Martino PB, Juárez MAT, Marçal DMS, Martins SCV, Ramalho JDC, DaMatta FM (2020) Elevated air [CO2] improves photosynthetic performance and alters biomass accumulation and partitioning in drought-stressed coffee plants. Environ Exp Bot 177:104137
Baba K (2006) Models of plant cell walls. In: Hayashi T (ed) The science and lore of the plant cell wall. Biosynthesis, structure and function. Brown Walker Press, Boca Raton, pp 3–10
Batista DS, Dias LLC, Rêgo MMD, Saldanha CW, Otoni WC (2017) Flask sealing on in vitro seed germination and morphogenesis of two types of ornamental pepper explants. Cien Rural 47:1–6
Bidhendi AJ, Geitmann A (2016) Relating the mechanics of the primary plant cell wall to morphogenesis. J Exp Bot 67:449–461
Castro KM, Batista DS, Fortini EA, Silva TD, Felipe SHS, Fernandes AM, Sousa RMJ, Nascimento LSQ, Campos VR, Grazul RM, Viccini LF, Otoni WC (2019) Photoperiod modulates growth, morphoanatomy, and linalool content in Lippia alba L. (Verbenaceae) cultured in vitro. Plant Cell Tissue Organ Cult 139:139–153
Cha-um S, Chanseetis C, Chitakovid W, Pichakum A, Supaibulwatana K (2011) Promoting root induction and growth of in vitro macadamia (Macadamia tetraphylla L. “Keaau”) plantlets using CO2-enriched photoautotrophic conditions. Plant Cell Tissue Organ Cult 106:435–444
Chen U-C, Nihsia C, Agrawal DC, Tsay H-S (2006) Influence of ventilation closures on plant growth parameters, acclimation and anatomy of leaf surface in Scrophularia yoshimurae Yamazaki - a medicinal plant native to Taiwan. Bot Stud 47:259–266
Clausen MH, Willats WGT, Knox JP (2003) Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr Res 338:1797–1800
Corrêa JPO, Vital CE, Pinheiro MVM, Batista DS, Azevedo JFL, Saldanha CW, Otoni WC (2015) In vitro photoautotrophic potential and ex vitro photosynthetic competence of Pfaffia glomerata (Spreng.) Pedersen accessions. Plant Cell Tissue Organ Cult 121:289–300
Corrêa JPO, Vital CE, Pinheiro MVM, Batista DS, Saldanha CW, Cruz ACF, Notini MM, Freitas DMS, DaMatta FM, Otoni WC (2016) Induced polyploidization increases 20-hydroxyecdysone content, in vitro photoautotrophic growth, and ex vitro biomass accumulation in Pfaffia glomerata (Spreng.) Pedersen. Vitro Cell Dev Biol Plant 52:45–55
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850
Cosgrove DJ (2014) Re-constructing our models of cellulose and primary cell wall assembly. Curr Opin Plant Biol 22:122–131
Cosgrove DJ (2018a) Nanoscale structure, mechanics and growth of epidermal cell walls. Curr Opin Plant Biol 46:77–86
Cosgrove DJ (2018b) Diffuse growth of plant cell walls. Plant Physiol 176:16–27
Costa AC, Rosa M, Megguer CA, Silva FG, Pereira FD, Otoni WC (2014) A reliable methodology for assessing the in vitro photosynthetic competence of two Brazilian savanna species: Hyptis marrubioides and Hancornia speciosa. Plant Cell Tissue Organ Culture 117:443–454
Cruz CD (2013) Genes—a software package for analysis in experimental statistics and quantitative genetics. Acta Scient Agron 35:271–276
Donaldson LA (2001) Lignification and lignin topochemistry—an ultrastructural view. Phytochemistry 57:859–873
Ellis M, Egelund J, Schultz CJ, Bacic A (2010) Arabinogalactan-proteins: key regulators at the cell surface? Plant Physiol 153:403–419
Ferreira PRB, Cruz ACF, Batista DS, Nery LA, Andrade IG, Rocha DI, Felipe SHS, Koehler AD, Nesi AN, Otoni WC (2019) CO2 enrichment and supporting material impact the primary metabolism and 20-hydroxyecdysone levels in Brazilian ginseng grown under photoautotrophy. Plant Cell Tissue Organ Cult 139:77–89
Fry SC (2001) Plant cell wall biosynthesis. eLS 1:2–8
Fujiwara K, Kozai T (1995) Physical microenvironment and its effects. In: Aitken-Christie J, Kozai T, Smith MAL (eds) Automation and environmental control in plant tissue culture. Kluwer Academic Publishers, Dordrecht, pp 319–369
Hassankhah A, Vahdati K, Lotfi M, Mirmasoumi M, Preece J, Assareh M-H (2014) Effects of ventilation and sucrose concentrations on the growth and plantlet anatomy of micropropagated Persian walnut plants. Int J Hortic Sci Technol 1:111–120
Hughes J, McCully ME (1975) The use of an optical brightener in the study of plant structure. Stain Technol 50:1037–1041
Iarema L, Cruz ACF, Saldanha CW, Dias LLC, Vieira RF, Oliveira EJ, Otoni WC (2012) Photoautotrophic propagation of Brazilian ginseng [Pfaffia glomerata (Spreng.) Pedersen]. Plant Cell Tissue Organ Cult 110:227–238
Kim JS, Daniel G (2012) Immunolocalization of hemicelluloses in Arabidopsis thaliana stem. Part I: temporal and spatial distribution of xylans. Planta 236:1275–1288. https://doi.org/10.1007/s00425-012-1686-y
Kirschbaum MU (2011) Does enhanced photosynthesis enhance growth? Lessons learned from CO2 enrichment studies. Plant Physiol 155:117–124
Kozai T (1991) Photoautotrophic micropropagation. In Vitro Cell Dev Biol Plant 27:47–51
Kozai T (2010) Photoautotrophic micropropagation—environmental control for promoting photosynthesis. Prop Ornam Plants 10:188–204
Kozai T, Kubota C (2005) Concepts, definitions, ventilation methods, advantages and disadvantages. In: Kozai T, Afreen F, Zobayed SMA (eds) Photoautotrophic (sugar-free medium) micropropagation as a new propagation and transplant production system. Springer, Dordrecht, pp 19–30
Kumar M, Campbell L, Turner S (2016) Secondary cell walls: biosynthesis and manipulation. J Exp Bot 67:515–531
Kumar S, Sreeharsha RV, Mudalkar S, Sarashetti PM, Reddy AR (2017) Molecular insights into photosynthesis and carbohydrate metabolism in Jatropha curcas grown under elevated CO2 using transcriptome sequencing and assembly. Sci Rep 7:11066
Lampugnani ER, Khan GA, Somssich M, Persson S (2018) Building a plant cell wall at a glance. J Cell Sci 131:jcs207373
Le Gall H, Philippe F, Domon JM, Gillet F, Pelloux J, Rayon C (2015) Cell wall metabolism in response to abiotic stress. Plants 4:112–166
Mamedes-Rodrigues TC, Batista DS, Napoleão TA, Fortini EA, Cruz ACF, Costa MGC, Otoni WC (2019) Regulation of cell wall development in Brachypodium distachyon in vitro as affected by cytokinin and gas exchange. Plant Cell Tissue Organ Cult 136:207–219
McCahill IW, Hazen SP (2019) Regulation of cell wall thickening by a medley of mechanisms. Trends Plant Sci 24:853–866
McCann MC, Knox JP (2011) Plant cell wall biology: polysaccharides in architectural and developmental contexts. Annu Plant Rev 41:343–366
McCartney L, Marcus SE, Knox JP (2005) Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J Histochem Cytochem 53:543–546
Moneo-Sánchez M, Alonso-Chico A, Knox JP, Dopico B, Labrador E, Martín I (2019) β-(1,4)-Galactan remodelling in Arabidopsis cell walls alters cellulose/xyloglucan interactions during cell elongation. Planta 249:351–362
Moneo-Sánchez M, Vaquero-Rodríguez A, Hernández-Nistal J, Albornos L, Knox P, Dopico B, Labrador E, Martín I (2020) Pectic galactan affects cell wall architecture during secondary cell wall deposition. Planta 251:100
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Neto AG, Silva-FilhoAA CJMLC, Vinholis AHC, Souza HB, Cunha WR, Silva MLAE, AlbuquerqueS BJK (2004) Evaluation of the trypanocidal and leishmanicidal in vitro activity of the crude hydroalcoholic extract of Pfaffia glomerata (Amarathanceae) roots. Phytomedicine 11:662–665
Nguyen QT, Xiao Y, Kozai T (2020) Photoautotrophic micropropagation. In: Kozai T, Niu G, Takagaki M (eds) Plant factory. Academic Press, New York, pp 333–346
Oliveira VF, Zaidan LBP, Braga MR, Aidar MPM, Carvalho MAM (2010) Elevated CO2 atmosphere promotes plant growth and inulin production in the cerrado species Vernonia herbacea. Funct Plant Biol 37:223–231
Pérez-Jiménez M, Bayo-Canha A, Lopez-Ortega G, Amor FM (2017) Growth, plant quality, and survival of sweet cherry (Prunus avium L.) seedlings are enhanced by CO2 enrichment. HortScience 52:1650–1654
Pérez-Jiménez M, López-Pérez AJ, Otálora-Alcón G, Marín-Nicolás D, Piñero MC, del Amor FM (2015) A regime of high CO2 concentration improves the acclimatization process and increases plant quality and survival. Plant Cell Tissue Organ Cult 121:547–557
Potocka I, Godel K, Dobrowolska I, Kurczyńska EU (2018) Spatio-temporal localization of selected pectic and arabinogalactan protein epitopes and the ultrastructural characteristics of explant cells that accompany the changes in the cell fate during somatic embryogenesis in Arabidopsis thaliana. Plant Physiol Biochem 127:573–589
Saldanha CW, Otoni CG, Notini MM, Kuki KN, Cruz ACF, Neto AR, Dias LLC, Otoni WC (2013) A CO2-enriched atmosphere improves in vitro growth of Brazilian ginseng [Pfaffia glomerata (Spreng.) Pedersen]. In Vitro Cell Dev Biol Plant 49:433–444
Saldanha CW, Otoni CG, Rocha DI, Cavatte PC, Detmann KSC, Tanaka FAO, Dias LLC, DaMatta FM, Otoni WC (2014) CO2-enriched atmosphere and supporting material impact the growth, morphophysiology and ultrastructure of in vitro Brazilian ginseng [Pfaffia glomerata (Spreng.) Pedersen] plantlets. Plant Cell Tissue Organ Cult 118:87–99
Sanches RFE, Catarino ICA, Braga MR, Silva EA (2017) Influência da alta concentração atmosférica de CO2 (↑[CO2]atm) × disponibilidade hídrica nas relações hídricas, trocas gasosas e acúmulo de carboidratos em Coffea arabica L. Hoehnea 44:635–643
Silva TD, Chagas K, Batista DS, Felipe SHS, Louback E, Machado LT, Fernandes AM, Buttrós VHT, Koehler AD, Farias LM, Santos AF, Silva PO, Otoni WC (2019) Morphophysiological in vitro performance of Brazilian ginseng (Pfaffia glomerata (Spreng.) Pedersen) based on culture medium formulations. In Vitro Cell Dev Biol Plant 55:454–467
Silva TD, Batista DS, Fortini EA, Castro KM, Felipe SHS, Fernandes AM, Sousa RMJ, Chagas K, Silva JVS, Correia LNF, Farias LM, Leite JPV, Rocha DI, Otoni WC (2020) Blue and red light affects morphogenesis and 20-hydroxyecdisone content of in vitro Pfaffia glomerata accessions. J Photochem Photobiol B 203:11761
Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T, Vorwerk S, Youngs H (2004) Toward a systems approach to understanding plant cell walls. Science 306:2206–2221
Tan L, Eberhard S, Pattathil S (2013) An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell 25:270–287
Thiem B, Kikowska M, Maliński MP, Kruszka D, Napierała M, Florek E (2017) Ecdysteroids: production in plant in vitro cultures. Phytochem Rev 16:603–622
Wei H, Jiqing Gou J, Yordanov Y, Zhang H, Thakur R, Wendy Jones W, Burton A (2013) Global transcriptomic profiling of Aspen trees under elevated [CO2] to identify potential molecular mechanisms responsible for enhanced radial growth. J Plant Res 126:305–320
Wolf S, Hematy K, Hofte H (2012) Growth control and cell wall signaling in plants. Annu Rev Plant Biol 63:381–407
Xiao Y, Niu G, Kozai T (2011) Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ Cult 105:149–158
Yates EA, Valdor J, Haslam SM, Morris HR, Dell A, Mackie W, Knox JP (1996) Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 6:131–139
Zhang Y, Brown G, Whetten R, Loopstra CA, Neale D, Kieliszewski MJ, Sederoff RR (2003) An arabinogalactan protein associated with secondary cell wall formation in differentiating xylem of loblolly pine. Plant Mol Biol 52:91–102
Zhu X, Xin X, Gu Y (2019) Cellulose and hemicellulose synthesis and their regulation in plant cells. In: Cohen E, Merzendorfer H (eds) Extracellular Sugar-Based. Biopolymers Matrices Biologically-Inspired Systems, vol 12. Springer, Cham, pp 303–353
Acknowledgements
The authors are also grateful to Dr. Paul Knox (University of Leeds, UK) for kindly providing the antibodies used and to the Núcleo de Microscopia e Microanálise (NMM) of the Universidade Federal de Viçosa for providing the facilities for the immunohistochemistry analysis. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by the Brazilian agencies Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Belo Horizonte, MG, Brazil; Grants Nos. PRONEX-CAG-APQ-01036-09; CRA-APQ-01651-13; CRA-BPD-00046-14: APQ-00772-19; and CRA–RED-00053-16/REDE MINEIRA - Estresse em Plantas to WCO and CBB-BPD-00020-16 to DSB), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brasília, DF, Brazil; Grant Finance Code 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasília, DF, Brazil: Grants Nos. MCT/CNPq 480675/2009-0; PQ 459.529/2014-5; and PQ 313901/2018-0 to WCO).
Author information
Authors and Affiliations
Contributions
EL, DSB, SHSF, TCM-Rand WCO designed the study; EL and TDS performed most of the experiments; EL, DSB, TDS and SHSF performed physiological and biochemical analyses; EL and TDS evaluated photosynthetic performance; EL and TARP performed histological studies; EL, TARP, DIR and DSB analyzed the data; EL, TARP, TCM-R, SHSF, DSB, DIR, PK, DS and WCO wrote the article with input from all other authors. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Consent for publication
All authors agree with the publication of the final manuscript.
Additional information
Communicated by Konstantin V. Kiselev.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Louback, E., Batista, D.S., Pereira, T.A.R. et al. CO2 enrichment leads to altered cell wall composition in plants of Pfaffia glomerata (Spreng.) Pedersen (Amaranthaceae). Plant Cell Tiss Organ Cult 145, 603–613 (2021). https://doi.org/10.1007/s11240-021-02031-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02031-4