Abstract
Although substantial progress has been made in the pathophysiology and management of the post-thrombotic syndrome (PTS), several aspects still need clarification. Among them, the incidence and severity of PTS in the real world, the risk factors for its development, the value of patient’s self-evaluation, and the ability to identify patients at risk for severe PTS. Eligible participants (n = 1107) with proximal deep-vein thrombosis (DVT) from the global GARFIELD-VTE registry underwent conventional physician’s evaluation for PTS 36 months after diagnosis of their DVT using the Villalta score. In addition, 856 patients completed a Villalta questionnaire at 24 months. Variable selection was performed using stepwise algorithm, and predictors of severe PTS were incorporated into a multivariable risk model. The optimistic adjusted c-index was calculated using bootstrapping techniques. Over 36-months, 27.8% of patients developed incident PTS (mild in 18.7%, moderate in 5.7%, severe in 3.4%). Patients with incident PTS were older, had a lower prevalence of transient risk factors of DVT and a higher prevalence of persistent risk factors of DVT. Self-assessment of overall PTS at 24 months showed an agreement of 63.4% with respect to physician’s evaluations at 36 months. The severe PTS multivariable model provided an optimistic adjusted c-index of 0.68 (95% CI 0.59–0.77). Approximately a quarter of DVT patients experienced PTS over 36 months after VTE diagnosis. Patient’s self-assessment after 24 months provided added value for estimating incident PTS over 36 months. Multivariable risk analysis allowed good discrimination for severe PTS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Incidence and severity of post-thrombotic syndrome (PTS) is lower than previously reported.
-
There is fair agreement between PTS self-assessment at 24 months and physician’s evaluation at 36 months.
-
A multivariable risk analysis allowed good discrimination for severe PTS.
Introduction
Post-thrombotic syndrome (PTS) is by far the most frequent long-term complication of deep-vein thrombosis (DVT) of the lower extremities. Based on the results of prospective cohort studies and randomized clinical trials, it is expected to occur in 40 to 50% of patients with a first episode of proximal DVT, and in 8–10% is serious enough to severely impair patient’s quality of life [1,2,3,4]. It is generally defined and classified according to the Villalta score [5,6,7].
Although substantial progress has been made in the pathophysiology and management of this long-term complication of DVT, several aspects still need clarification [8]. Among them, the incidence and severity of PTS in the real world, the risk factors for its development, the ability to predict the development of severe PTS, and the value of patient’s self-evaluation, particularly desirable in circumstances like the current pandemic.
Global Anticoagulant Registry in the FIELD-Venous Thromboembolism (GARFIELD-VTE) is a prospective, non-interventional, global registry of over 10,000 patients with VTE recruited worldwide [9]. As in an unselected subgroup of patients referring with DVT, careful information on the long-term development of PTS was collected, we had the opportunity to address some clinically relevant questions that are still unanswered.
The main purposes of our investigation were: to determine the 36-month incidence of overall and severe PTS in a broad series of unselected patients belonging to the GARFIELD-VTE registry; to assess the risk factors of PTS development; to assess the value of patient’s self-evaluation via the Villalta score after 24 months; and to test the discriminatory ability of a multivariable model for the risk of severe PTS. To ensure these findings were comparable with previously published data, only patients with proximal DVT were included in the study analysis.
Materials and methods
GARFIELD-VTE included more than 10,000 registered participants with recent diagnosis of VTE over a 3-year period and collected prospective data on clinical outcomes, treatment patterns and risk factors. The registry was funded by an unrestricted research grant from Bayer AG, and the ongoing work is supported by the Thrombosis Research Institute. At the end of the follow-up period, in patients recruited for lower limb DVT, alone or associated with pulmonary embolism (PE), physicians who had agreed a priori on this program were asked to make a PTS assessment based on the Villalta score. The presence of five leg symptoms (i.e. pain, cramps, heaviness, paresthesia and pruritus) and six objective signs (i.e. pretibial edema, induration of the skin, hyperpigmentation, redness, venous ectasia, and pain during calf compression) was scored. For each item, a score of 0 (not present) up to 3 (most severe) was assigned, using the contralateral unaffected leg as reference. The presence of a score of 0 to 4 indicated no PTS, 5 to 9, mild PTS, and 10 to 14, moderate PTS, while a score of 15 or more or the presence of skin ulcer indicated severe PTS [5].
One year earlier, a patient’s self-assessment was scheduled in consenting subjects with the help of a questionnaire (sent by mail) enquiring the presence and severity of the subjective symptoms and objective findings captured by the Villalta score (all but skin ulcer). For this purpose, the 11 parameters of the Villalta Score were translated into lay language and given to patients for self-examination and answering the questions (Table 1). For each complaint, patients were requested to quantify the burden using a Likert scale from 0 (not present) to 10 (most severe). Each question in the questionnaire was converted to the original score using the following rule: 0 = absent; 1 to 3 = 1 point; 4 to 7 = 2 points; 8 to 11 = 3 points. An additional question enquired the pre-existence of complaints before the DVT episode. If this was the case, the related sign or symptom was only computed if according to the patient’s opinion it had significantly worsened. To be consistent with the Villalta score, the presence of an overall score of 5 to 9 indicated mild PTS, of 10 to 14 moderate PTS, while a score of 15 or more indicated severe PTS. The original Villalta score can be found in the supplementary material (Supplementary Table 1).
Contingency tables comparing the frequency distribution of the derived PTS diagnosis (none, mild, moderate, severe) were created. The agreement between the patient’s self-assessment and the physician assessment was quantified by the proportion of identical PTS diagnosis from the total.
To test the discriminatory ability of a multivariable model for the risk of severe PTS, a multivariable logistic regression model was calculated according to standard methods. The patient variables at baseline to be included in the model were the following: (1) patient demographics: age, gender, ethnicity, BMI and smoking, (2) treatment modalities (conventional and direct oral anticoagulants); (3) the prescription of elastic compression stockings; (4) provoking factors of VTE (within 3 months before diagnosis): acute medical illness, hospitalization, long-haul travelling, trauma of the lower limb, surgery, and active cancer; (5) special risk factors associated with VTE: active cancer; (6) predisposing risk factors: chronic heart failure, chronic immobilization, family history of VTE, history of cancer, known thrombophilia, prior episode of DVT, and renal insufficiency.
The Area Under the Receiver Operating Characteristic (ROC) curve (AUC) was calculated as a measure of the ability of the model to distinguish between patients who developed PTS from those that did not. A calibration plot displaying the predicted event rate against the observed rates was constructed. The predicted event rates were divided into quintiles and the mean event rate in each quintile was calculated and plotted against the mean observed event rate in each decile. Finally, the discrimination performance of the final model was evaluated through c-index (AUC) by adjusting for optimism using bootstrapping. The analysis was conducted using the statistical programme SAS.
Results
Patients and development of PTS
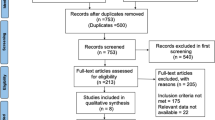
Of the 10 679 patients with acute VTE that were recruited in the GARFIELD-VTE registry between May 2014 and January 2017, 2457 were excluded because of clinical presentation with isolated PE. A further 436 were excluded due to upper limb DVT (N = 436), caval vein thrombosis (N = 149), isolated distal DVT (N = 1227), died during the study enrolment (N = 1175), or lacked of end-of-study physician assessment (N = 4082). A final 28 participants were excluded due to inconsistent data provided. Accordingly, 1107 patients were recruited in the current investigation. Based on the Villalta score, PTS was detected in 308 patients (27.8%), and was mild in 207 (18.7%), moderate in 63 (5.7%), and severe in the remaining 38 (3.4%) (Fig. 1).
Risk factors of PTS
The main demographic and clinical characteristics of the recruited patients including their persistent and provoking risk factors are shown in Table 2. Patients with PTS were on average older and were more likely to be Caucasian. Patients with PTS also had a lower prevalence of long-haul travelling among the provoking risk factors; and were more likely to have chronic heart failure and chronic immobilization among the predisposing risk factors.
Patient’s self-assessment
The self-assessment of overall PTS at 24 months was available in 856 of the 1107 patients (77%) patients. As shown in Table 3 with respect to physician’s evaluation at 36 months, patient’s self-assessment at 24 months had an agreement of 63.4% with a weighted kappa of 0.37 (0.31,0.42). The median (Q1, Q2) and Mean (SD) Villalta score for patients’ self-assessment at 24 months and physician’s assessment at 36 months have been shown in Fig. 2.
Risk model of severe PTS
Figure 3 (see Supplementary Table 2 for full results) shows the results of the multivariable analysis that was conducted to identify parameters associated with the risk of severe PTS. As shown in the figure, chronic heart failure, chronic immobilization, and prior episode of DVT were found to be independent predictors of severe PTS. The multivariable model provided good discrimination for severe PTS over 36-months, yielding an optimistic adjusted c-index of 0.68 (95% CI 0.59 to 0.77) (Fig. 4).
Discussion
The management of PTS can be both time-consuming and frustrating for clinicians. Once established, especially when complicated with leg ulceration, it is a significant cause of disability and economic burden for patients and healthcare systems alike [10, 11].
Proximal DVT is known to be associated with a higher frequency and more severe PTS than distal DVT [2]. Typically studies in PTS have focused on these patients and for the results to this analysis to be comparable to similar studies only patients with proximal DVT were included. The key findings of this analysis which includes both proximal and distal DVT patients have previously been presented in ISTH 2021 [12].
Based on the experience gained in contemporary years in the framework of the international GARFIELD-VTE registry, the rate of both overall and severe PTS, as assessed by physicians 3 years after the thrombotic episode, seems remarkably lower than that reported in past prospective cohorts and randomized clinical trials [1, 2]. Indeed, overall PTS was found in approximately one quarter of patients (as compared to 40 to 50%), and that of the most severe manifestations in under 4% (as compared to 8 to 10%). Of course, the heterogeneity of data collection as performed by untrained physicians in the real world can account at least partially for this discrepancy. When examining the change in patient self-reported Villalta from 6 to 24 months a 63% agreeance between time frames was observed. This is a similar level of agreeance we see between patients with a 24-month and 36-month physician score. It is important to note that differences in individual categories of PTS (none, moderate, mild, severe) will have more variability than observing binary presence or absence of PTS. However, the fair correspondence between the physician’s assessment and the patient’s self-evaluation demonstrated by the weighted Kappa of 0.3664 (0.31, 0.42) makes these findings reliable. It should be considered that referral of patients with suspected DVT to specialists and the diagnostic process has seen major improvements over time, as documented by a shortening of patient-doctor delay and the replacement of high-threshold, invasive contrast venography by non-invasive and easily accessible ultrasonography, besides the improved initial and long-term treatment of DVT, including the replacement of VKA with the novel DOACs and the refinement of long-term anticoagulation strategies. Hence, it is likely that in recent years the thrombotic burden of patients with DVT has considerably reduced in comparison to older studies.
Patients with PTS were on average older and had a higher prevalence of prior episodes of VTE. They also had a lower prevalence of transient risk factors of DVT and were more likely to have persistent risk factors of DVT, such as chronic heart failure and chronic immobilization. It is also worth noting that special patient populations such as those that have renal insufficiency or a history of cancer did not appear more likely to have PTS. It is possible that this may be due to the low patient numbers and poorer survival of these patients, however further investigation in these patient populations may be warranted. These findings are consistent with those coming from most contemporary studies [6, 7]. Some of the risk factors detailed in this analysis have not been commonly mentioned in previous studies but recorded in the GARFIELD-VTE registry after consultation from clinical experts in the field. Clinical risk factors that have been commonly associated with increased risk of PTS include obesity, chronic heart failure and a history of VTE [13,14,15], however in another large VTE registry (COMMAND-VTE) chronic kidney disease, leg swelling, and active cancer were independent risk factors for PTS [16].
For detecting and grading the severity of PTS we adopted the Villalta score, which among available tools is by far the most widely used and recommended by scientific societies [5]. Diagnosis of PTS by the Villalta score requires a clinical visit to perform a physical examination of the affected limb, which represents a challenge and limits the possibility of its use when conducting large studies as well as in several circumstances, such as the current pandemic. Furthermore, this may be considered an interventional procedure as it requires an examination of the patient that is not normally performed in routine patient care and is therefore not suitable for prospective non-interventional registry studies. While a tool for patient reporting of the Villalta score would reduce health resource utilization and the burden imposed on patients participating in clinical trials, the questionnaires so far developed and validated for patient self-assessment require a visually assisted form [5, 17]. In our project, a patient’s self-evaluation was obtained after 2 years with the help of a questionnaire enquiring the presence and severity of subjective symptoms and objective findings (all but skin ulcer). Although all items were completed by the patient without any visual support, the self-assessment of overall PTS at 24-months was found to be accurate (accuracy, 0.74; 95% CI 0.71 to 0.76) with respect to the physician’s 36-month evaluation, with a good sensitivity and specificity (66 and 77%, respectively). Hence, our findings have the potential to provide clinicians with a new useful tool for detecting PTS and quantifying its severity without the aid from health-care personnel.
Although in recent years a few scores have been identified and validated that can help predict the development of PTS [18,19,20,21], their value is uncertain, as is their potential implications for clinical practice [7]. Based on the results of the multivariable analysis we have conducted, chronic heart failure and chronic immobilization were found to be statistically significant independent predictors of severe PTS, while there was a weak association between the use of compression stockings and reduced likelihood of PTS. This simple and practical multivariable model provided good discrimination for severe PTS over 36-months, yielding an optimistic adjusted c-index of 0.61 (95% CI 0.58 to 0.64). Accordingly, it has the potential to help predict the development of severe PTS even in the hands of less experienced physicians. However, its implementation in clinical practice requires external validation from additional cohorts of patients.
Our investigation presents several limitations that deserve careful attention. The clinical examination regarding PTS was conducted by physicians who represent the standard of care in the respective countries, but who, in contrast to randomised clinical trials, were not specifically trained for the PTS examination. In order to prevent misinterpretation of clinical signs, the reporting of skin ulcer was not included in the questionnaire addressing the patient’s self-assessment of PTS. Furthermore, additional well-known risk factors of PTS, such as the ilio-femoral location of thrombosis and the presence of varicose veins or other manifestations of chronic venous insufficiency were not captured by the registry, nor was the development of ultrasound detectable manifestations of vascular damage (residual vein thrombosis or popliteal valve reflux). Since heart failure is associated with an increased risk of PTS another limitation that should be noted is that there is a risk that venous insufficiency and heart failure symptoms could be misdiagnosed as PTS.
For logistic reasons, it was not possible to obtain a patient’s self-assessment of PTS after 3 years, i.e. at the same time of the physician’s assessment. However, it is well known that most PTS complications develop within the first 2 years of the thrombotic episode [6]. Finally, the prescription of elastic stockings was left to discretion of the investigator and no information is available on the type and duration of these devices.
In conclusion, our findings suggest that the incidence and severity of PTS in the real world is lower than that reported in contemporary studies addressing the development of this complication. The patient self-assessment we described for the first time using the Villalta score at 24 months can be used to simply estimate the risk of PTS over 36 months. A simple multivariate risk model, based on easily accessible information at patient’s presentation provided good discriminatory power for predicting severe PTS risk over 36 months after DVT diagnosis.
Data Availability
Aggregated data can be shared upon reasonable request and analysis plan to Saverio Virdone (Svirdone@tri-london.ac.uk).
References
Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E, Prins MH (1996) The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 125:1–7
Kahn SR, Shrier I, Julian JA, Ducruet T, Arsenault L, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, Desjardins L, Lamping DL, Johri M, Ginsberg JS (2008) Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 149:698–707
Kahn SR, Shapiro S, Wells PS, Rodger MA, Kovacs MJ, Anderson DR, Tagalakis V, Houweling AH, Ducruet T, Holcroft C, Johri M, Solymoss S, Miron MJ, Yeo E, Smith R, Schulman S, Kassis J, Kearon C, Chagnon I, Wong T, Demers C, Hanmiah R, Kaatz S, Selby R, Rathbun S, Desmarais S, Opatrny L, Ortel TL, Ginsberg JS, SOX trial investigators (2014) Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet 383:880–888
Amin EE, Ten Cate-Hoek AJ, Bouman AC, Meijer K, Tick L, Middeldorp S, Mostard G, Ten Wolde M, van den Heiligenberg S, van Wissen S, van de Poel M, Villalta S, Serné E, Otten HM, Klappe E, Prandoni P, Prins MH, Ten Cate H, Joore MA (2018) Individually shortened duration versus standard duration of elastic compression therapy for prevention of post-thrombotic syndrome: a cost-effectiveness analysis. Lancet Haematol 5:e512–e519
Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis (2009) Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost 7:879–883
Kahn SR, Comerota AJ, Cushman M, Evans NS, Ginsberg JS, Goldenberg NA, Gupta DK, Prandoni P, Vedantham S, Walsh ME, Weitz JI, American Heart Association Council on Peripheral Vascular Disease, Council on Clinical Cardiology, and Council on Cardiovascular and Stroke Nursing (2014) The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation 130:1636–1661
Visonà A, Quere I, Mazzolai L, Amitrano M, Lugli M, Madaric J, Prandoni P (2021) European Society of Vascular Medicine (ESVM). Post-thrombotic syndrome. Vasa 50:331–340
Tritschler T, Kraaijpoel N, Le Gal G, Wells PS (2018) Venous thromboembolism: advances in diagnosis and treatment. JAMA 320:1583–1594
Ageno W, Haas S, Weitz JI, Goldhaber SZ, Turpie AGG, Goto S, Angchaisuksiri P, Nielsen JD, Kayani G, Pieper KS, Schellong S, Bounameaux H, Mantovani LG, Prandoni P, Kakkar AK, GARFIELD-VTE investigators (2019) Characteristics and management of patients with venous thromboembolism: the GARFIELD-VTE Registry. Thromb Haemost 119:319–327
Bergqvist D, Jendteg S, Johansen L, Persson U, Ödegaard K (1997) Cost of long term complications of deep venous thrombosis of the lower extremities: an analysis of a defined patient population in Sweden. Ann Intern Med 126:454–457
Kahn SR, M’Lan CE, Lamping DL, Kurz X, Bérard A, Abenhaim L (2004) The influence of venous thromboembolism on quality of life and severity of chronic venous disease. J Thromb Haemost 2:2146–2151
Prandoni P, Farjat AE, Haas S, Turpie AG, Bounameaux H, Schellong S, Goldhaber SZ, Kayani G, Carrier M, Jacobson B, Ageno W, Weitz JI, Goto S, Angchaisuksiri P, Mantovani LG, Kakkar AK (2021) Incidence and predictors of post-thrombotic syndrome: results from GARFIELD-VTE [abstract]. Res Pract Thromb Haemost 5(Suppl 2)
Spiezia L, Campello E, Simion C, Poretto A, Dalla Valle F, Simioni P (2022) Risk factors for post-thrombotic syndrome in patients with a first proximal deep venous thrombosis treated with direct oral anticoagulants. Angiology 73(7):649–654. https://doi.org/10.1177/00033197211070889
Cucuruz B, Kopp R, Pfister K, Noppeney J, Tripal K, Korff T, Zeman F, Koller M, Noppeney T (2020) Risk and protective factors for post-thrombotic syndrome after deep venous thrombosis. J Vasc Surg Venous Lymphat Disord 8(3):390–395. https://doi.org/10.1016/j.jvsv.2019.10.012
Ende-Verhaar YM, Tick LW, Klok FA, Huisman MV, Rosendaal FR, le Cessie S, Cannegieter SC (2019) Post-thrombotic syndrome: short and long-term incidence and risk factors. Thromb Res 177:102–109. https://doi.org/10.1016/j.thromres.2019.03.003
Nishimoto Y, Yamashita Y, Morimoto T, Saga S, Amano H, Takase T, Hiramori S, Kim K, Oi M, Akao M, Kobayashi Y, Toyofuku M, Izumi T, Tada T, Chen PM, Murata K, Tsuyuki Y, Sasa T, Sakamoto J, Kinoshita M, Togi K, Mabuchi H, Takabayashi K, Shiomi H, Kato T, Makiyama T, Ono K, Sato Y, Kimura T, COMMAND VTE Registry Investigators (2019) Risk factors for post-thrombotic syndrome in patients with deep vein thrombosis: from the COMMAND VTE registry. Heart Vessels. 34(4):669–677. https://doi.org/10.1007/s00380-018-1277-3
Utne KK, Ghanima W, Foyn S, Kahn S, Sandset PM, Wik HS (2016) Development and validation of a tool for patient reporting of symptoms and signs of the post-thrombotic syndrome. Thromb Haemost 115:361–367
Rabinovich A, Ducruet T, Kahn SR, SOX Trial investigators (2018) Development of a clinical prediction model for the postthrombotic syndrome in a prospective cohort of patients with proximal deep vein thrombosis. J Thromb Haemost 16:262–270
Rabinovich A, Gu CS, Vedantham S, Kearon C, Goldhaber SZ, Gornik HL, Kahn SR, ATTRACT Trial Investigators (2020) External validation of the SOX-PTS score in a prospective multicenter trial of patients with proximal deep vein thrombosis. J Thromb Haemost 18:1381–1389
Amin EE, van Kuijk SMJ, Joore MA, Prandoni P, Cate HT, Cate-Hoek AJT (2018) Development and validation of a practical two-step prediction model and clinical risk score for post-thrombotic syndrome. Thromb Haemost 118:1242–1249
Zhang J, Ma F, Yao J, Hao B, Xu H, Guo X, Gao H, Yang T (2022) Development and validation of a clinical prediction model for post thrombotic syndrome following anticoagulant therapy for acute deep venous thrombosis. Thromb Res 214:68–75
Acknowledgements
All authors were involved in reviewing the manuscript. Manuscript drafting assistance was provided by Shakirah Chowdhury (Thrombosis Research Institute, London, UK). SAS programming support was provided by Uma Maheshwari (Thrombosis Research Institute, London, UK).
Funding
This work was supported by the Thrombosis Research Institute (London, UK).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Paolo Prandoni: Personal fees from Bayer Pharma AG, Pfizer, Daiichi-Sankyo, Italfarmaco, Alfasigma and Sanofi. Sylvia Haas: Honoraria from Bayer Pharma AG, Bristol Myers Squibb, Daiichi-Sankyo, Pfizer, Portola, Sanofi. Sebastian Schellong: Speaker fees from Bayer Pharma AG, Boehringer-Ingelheim, Bristol Meyer Squibb, Daiichi-Sankyo, Sanofi Aventis and Pfizer. Consultancy fees from Bayer Pharma AG, Boehringer-Ingelheim, Daiichi-Sankyo, Sanofi Aventis, Aspen and Pfizer. Harry Gibbs: Personal fees from Pfizer, Bayer, Boehringer Ingelheim, and Eli Lilly. Peter Verhamme: Research support and honoraria from Bayer Healthcare, Boehringer, Daiichi-Sankyo, Anthos Pharmaceuticals, Portola, Leo-Pharma, BMS and Pfizer. Marc Carrier: Grants from Pfizer/BMS, Canadian Institutes of Health Research, grants and personal fees from Leo Pharma, Bayer, personal fees from Sanofi Aventis, Pfizer, Bristol Myers Squibb. Barry Jacobson: Personal fees from Bayer HealthCare, Sanofi-Aventis. J.I.A. reports speaker fees from Sanofi, Rovi, Bayer AG, and Aspen. Hugo ten Cate: research support from Bayer, consultant for Alveron. Shareholder Coagulation Profile. Elizaveta Panchenko: Research support from Pfizer, Bristol-Myers Squibb Boehringer Ingelheim, Sanofi, AstraZeneca, Daiichi Sankyo Pharma Development, GlaxoSmithKline DMPK. Speaker fees from Sanofi, Takeda-NYCOMED, Boehringer Ingelheim, Pfizer, Bristol-Myers Squibb, Bayer, Lilly, AstraZeneca, GlaxoSmithKline, Servier. Consultancy fees from Sanofi, Bayer, Lilly, AstraZeneca; Boehringer Ingelheim, Bayer, Pfizer, Bristol-Myers Squibb, Lilly, Servier Peter Verhamme: Research support and honoraria from Bayer Healthcare, Boehringer, Daiichi-Sankyo, Anthos Pharmaceuticals, Portola, Leo-Pharma, BMS and Pfizer. Karen Pieper: Consultancy fee from Johnson & Johnson. Professor Ajay K Kakkar: Research grants from Bayer Pharma AG and Sanofi. Personal fees from Anthos Therapeutics, Bayer Pharma AG, Sanofi S.A. Meg E Fluharty, Gloria Kayani, Eric Tse: None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A full list of investigators is given in the Online Appendix.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prandoni, P., Haas, S., Fluharty, M.E. et al. Incidence and predictors of post-thrombotic syndrome in patients with proximal DVT in a real-world setting: findings from the GARFIELD-VTE registry. J Thromb Thrombolysis 57, 312–321 (2024). https://doi.org/10.1007/s11239-023-02895-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-023-02895-7