Abstract
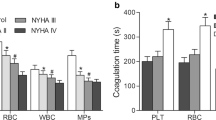
Patients with severe aortic stenosis (AS) after replacement of the transcatheter aortic valve (TAVR) are more likely to develop thrombotic complications such as cerebral embolism and artificial valve thrombosis. However, the mechanism is not yet well defined. We aimed to explore the plasma extracellular vesicles (EVs) levels and their role in the induction of procoagulant activity (PCA) in patients receiving TAVR alone or TAVR with percutaneous coronary intervention (PCI). EVs were analyzed with flow cytometer. Markers of platelet and endothelial cell activation were quantified using selective enzyme-linked immunosorbent assay (ELISA) kits. Procoagulant activity (PCA) was assessed by clotting time, purified clotting complex assays, and fibrin production assays. Our results confirmed that EVs with positive phosphatedylserin (PS+EV), platelet EVs (PEVs) and positive tissue factor EVs (TF+EVs) were higher in patients following TAVR than before TAVR, particularly in TAVR with PCI. Furthermore, endothelial-derived EVs (EEVs) were also higher in patients after TAVR with PCI than pre-TAVR, however, the EEVs levels in TAVR alone patients were gradually reduce than pre-TAVR. In addition, we further proved that total EVs contributed to dramatically shortened coagulation time, increased intrinsic/extrinsic factor Xa and thrombin generation in patients after TAVR, especially in TAVR with PCI. The PCA was markedly attenuated by approximately 80% with lactucin. Our study reveals a previously unrecognized link between plasma EV levels and hypercoagulability in patients after TAVR, especially TAVR with PCI. Blockade of PS+EVs may improve the hypercoagulable state and prognosis of patients.
Highlights
-
Patients after TAVR, especially TAVR with PCI, suffer from increased thrombotic complications, which seriously affect the prognosis of the patients.
-
Total EVs levels tend to increase after TAVR, especially TAVR with PCI, in 6 months, which positive correlation with the hypercoagulable state, and may lead to thrombosis.
-
EVs play the procoagulant role in patients after TAVR, especially TAVR with PCI.
-
Blockade of PS-positive EVs (PS+EVs) may improve the hypercoagulable state and reduce thrombotic complications in patients after TAVR.




Similar content being viewed by others
References
Hu PP (2012) TAVR and SAVR: current treatment of aortic stenosis. Clin Med Insights Cardiol 6:125–139
Yutzey KE, Demer LL, Body SC et al (2014) Calcific aortic valve disease: a consensus summary from the Alliance of investigators on calcific aortic valve disease. Arterioscler Thromb Vasc Biol 34:2387–2393
Cappabianca G, Ferrarese S, Musazzi A et al (2016) Predictive factors of long-term survival in the octogenarian undergoing surgical aortic valve replacement: 12-year single-centre follow-up. Heart Vessels 31:1798–1805
Mastoris I, Schoos MM, Dangas GD et al (2014) Stroke after transcatheter aortic valve replacement: incidence, risk factors, prognosis, and preventive strategies. Clin Cardiol 37:756–764
Auffret V, Regueiro A, Trigo MD et al (2016) Predictors of early cerebrovascular events in patients with aortic stenosis undergoing transcatheter aortic valve replacement. J Am Coll Cardiol 68:673–684
Yanagisawa R, Hayashida K, Yamada Y et al (2016) Incidence, predictors, and mid-term outcomes of possible leaflet thrombosis after TAVR. JACC Cardiovasc Imaging
Brouwer J, Nijenhuis VJ, Delewi R et al (2020) Aspirin with or without Clopidogrel after Transcatheter aortic-valve implantation. N Engl J Med 383:1447–1457
Nijenhuis VJ, Brouwer J, Delewi R et al (2020) Anticoagulation with or without Clopidogrel after Transcatheter aortic-valve implantation. N Engl J Med 382:1696–1707
Yeung T, Gilbert GE, Shi J et al (2008) Membrane phosphatidylserine regulates surface charge and protein localization. Science 319:210–213
Owens AP 3rd, Mackman N (2011) Microvesicles in hemostasis and thrombosis. Circ Res 108:1284–1297
Mackman N, Tilley RE, Key NS (2007) Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol 27:1687–1693
Perez-Pujol S, Marker PH, Key NS (2007) Platelet microvesicles are heterogeneous and highly dependent on the activation mechanism: studies using a new digital flow cytometer. Cytometry A 71:38–45
Carroll JD, Mack MJ, Vemulapalli S et al (2020) STS-ACC TVT Registry of Transcatheter aortic valve replacement. J Am Coll Cardiol 76:2492–2516
Jung C, Lichtenauer M, Figulla H-R et al (2017) Microvesicles in patients undergoing transcatheter aortic valve implantation (TAVI). Heart Vessels 32:458–466
Zhou B, Li J, Chen S et al (2016) Time course of various cell origin circulating microvesicles in ST-segment elevation myocardial infarction patients undergoing percutaneous transluminal coronary intervention. Exp Ther Med 11:1481–1486
Jung RG, Duchez A, Simard T et al (2022) Plasminogen activator inhibitor-1-Positive platelet-derived extracellular vesicles predicts MACE and the Proinflammatory SMC phenotype. JACC Basic Transl Sci 7:985–997
Joseph J, Naqvi SY, Giri J et al (2017) Aortic stenosis: pathophysiology, diagnosis, and Therapy. Am J Med 130:253–263
Shi J, Heegaard CW, Rasmussen JT et al (2004) Lactadherin binds selectively to membranes containing phosphatidyl-L-serine and increased curvature. Biochim Biophys Acta 1667:82–90
Marchini JF, Miyakawa AA, Tarasoutchi F et al (2016) Endothelial, platelet, and macrophage microparticle levels do not change acutely following transcatheter aortic valve replacement. J Negat Results Biomed 15:7
He Z, Si Y, Jiang T et al (2016) Phosphotidylserine exposure and neutrophil extracellular traps enhance procoagulant activity in patients with inflammatory bowel disease. Thromb Haemost 115:738–751
Horn P, Stern D, Veulemans V et al (2015) Improved endothelial function and decreased levels of endothelium-derived microvesicles after transcatheter aortic valve implantation. EuroIntervention 10:1456–1463
Petrov G, Regitz-Zagrosek V, Lehmkuhl E et al (2010) Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation 122:23–28
Li J, Tong D, Chen F et al (2021) Inflammatory cytokines enhance procoagulant activity of platelets and endothelial cells through phosphatidylserine exposure in patients with essential hypertension. J Thromb Thrombolysis 51:933–940
Atmaca HU, Akbas F, Aral H (2019) Relationship between circulating microvesicles and hypertension and other cardiac disease biomarkers in the elderly. BMC Cardiovasc Disord 19:164
Shaw AW, Pureza VS, Sligar SG et al (2007) The local phospholipid environment modulates the activation of blood clotting. J Biol Chem 282:6556–6563
Rao LVM, Kothari H, Pendurthi UR (2012) Tissue factor encryption and decryption: facts and controversies. Thromb Res 129:13–17
Koganti S, Eleftheriou D, Gurung R et al (2021) Persistent circulating platelet and endothelial derived microparticle signature may explain on-going pro-thrombogenicity after acute coronary syndrome. Thromb Res 206:60–65
Biasucci LM, Porto I, Di Vito L et al (2012) Differences in microparticle release in patients with acute coronary syndrome and stable angina. Circ J 76:2174–2182
Lacroix R, Dignat-George F (2012) Microvesicles as a circulating source of procoagulant and fibrinolytic activities in the circulation. Thromb Res 129:27–29
Lacroix R, Plawinski L, Robert S et al (2012) Leukocyte- and endothelial-derived microvesicles: a circulating source for fibrinolysis. Haematologica 97:1864–1872
Vion AC, Ramkhelawon B, Loyer X et al (2013) Shear stress regulates endothelial microparticle release. Circ Res 112:1323–1333
Nomura S, Tandon NN, Nakamura T et al (2001) High-shear-stress-induced activation of platelets and microvesicles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis 158:277–287
Nomura S, Komiyama Y (1997) Shear stress and platelet-derived microvesicles. Rinsho Byori 45:927–933
Ding J, Chen Z, Niu S et al (2015) Quantification of Shear-Induced platelet activation: high shear stresses for short exposure time. Artif Organs 39:576–583
Giannella A, Ceolotto G, Radu CM et al (2021) PAR-4/Ca(2+)-calpain pathway activation stimulates platelet-derived microvesicles in hyperglycemic type 2 diabetes. Cardiovasc Diabetol 20:77
Nomura S, Imamura A, Okuno M et al (2000) Platelet-derived microvesicles in patients with arteriosclerosis obliterans: enhancement of high shear-induced microparticle generation by cytokines. Thromb Res 98:257–268
Leroyer AS, Ebrahimian TG, Cochain C et al (2009) Microvesicles from ischemic muscle promotes postnatal vasculogenesis. Circulation 119:2808–2817
Karten B, Campenot RB, Vance DE et al (2006) Expression of ABCG1, but not ABCA1, correlates with cholesterol release by cerebellar astroglia. J Biol Chem 281:4049–4057
Komoriyama H, Kimiya K, Nagai T et al (2021) Blood flow dynamics with four-dimensional flow cardiovascular magnetic resonance in patients with aortic stenosis before and after transcatheter aortic valve replacement. J Cardiovasc Magn Reson 23:81
Leroyer AS, Isobe H, Leseche G et al (2007) Cellular origins and thrombogenic activity of microvesicles isolated from human atherosclerotic plaques. J Am Coll Cardiol 49:772–777
Viera AJ, Mooberry M, Key NS (2012) Microvesicles in cardiovascular disease pathophysiology and outcomes. J Am Soc Hypertens 6:243–252
Barthélémy O, Collet JP, Montalescot G (2016) Cerebral embolism: a silent iatrogenic complication of TAVR that needs voiced consideration. J Am Coll Cardiol 68:600–602
Nombela-Franco L, Webb JG, Jaegere PP et al (2012) Timing, predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation 126:3041–3053
Ghanem A, Kocurek J, Sinning J-M et al (2013) Cognitive trajectory after transcatheter aortic valve implantation. Circ Cardiovasc Interv 6:615–624
Pache G, Schoechlin S, Blanke P et al (2016) Early hypo-attenuated leaflet thickening in balloon-expandable transcatheter aortic heart valves. Eur Heart J 37:2263–2271
Holmes DR Jr, Mack MJ, Kaul S et al (2012) 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 59:1200–1254
Acknowledgements
We thank FX for blood sample collection; and JZ and DT for excellent technical assistance.
Funding
This study was supported by the Medical and Health Science and Technology Development Plan Project of Shandong Province (202003010399).
Author information
Authors and Affiliations
Contributions
DT and JL conceived the study. HC, FX, GZ and JZ conducted the assays. GJ, YS and HC collected and analyzed the data, and made the tables and figures, wrote and retouch the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Authors, HC, YS, FX, GZ, JZ, GJ, DT and JL declare to have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chi, H., Shao, Y., Xie, F. et al. Procoagulant effect of extracellular vesicles in patients after transcatheter aortic valve replacement or transcatheter aortic valve replacement with percutaneous coronary intervention. J Thromb Thrombolysis 56, 264–274 (2023). https://doi.org/10.1007/s11239-023-02835-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-023-02835-5