Abstract
Background
Standard modifiable risk factors (SMuRFs) increase the risk of cardiovascular events in patients with acute coronary syndrome (ACS) and are also strongly associated with obstructive sleep apnea (OSA) in a bidirectional relationship. However, the association of OSA with recurrent cardiovascular events in ACS patients based on the number of SMuRFs remains unclear. Hence, we aimed to elucidate the prognostic implication of OSA in ACS patients stratified by the number of SMuRFs.
Methods
This was a post hoc analysis of the OSA-ACS study (NCT03362385), including 1927 patients admitted for ACS and undergoing portable sleep monitoring. OSA was defined as an apnea hypopnea index ≥ 15 events/h. The primary endpoint was major adverse cardiovascular and cerebrovascular event (MACCE) including cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina or heart failure, and ischemia-driven revascularization. Cox proportional hazards model and Kaplan-Meier analysis were used to investigated the relationship between OSA and subsequent cardiovascular events after patients were stratified by the number of SMuRFs.
Results
Among 1927 patients enrolled, 130 (6.7%) had no SMuRF, 1264 (65.6%) exhibited 1–2 SMuRFs and 533 (27.7%) presented 3–4 SMuRFs. With the increase of the number of SMuRFs, the proportion of OSA in ACS patients tended to increase (47.7% vs. 51.5% vs. 56.6%), but there was no significant difference between them (P = 0.08). After the stratification of ACS patients via SMuRF numbers and adjustment for confounding factors, fully adjusted Cox regression indicated that OSA increased the risk of MACCE (adjusted HR, 1.65; 95%CI, 1.06–2.57; P = 0.026) and ischemia-driven revascularization (adjusted HR, 2.18; 95%CI, 1.03–4.65; P = 0.042) in ACS patients with 3–4 SMuRFs.
Conclusions
In hospitalized ACS patients, OSA is associated with an increased risk of MACCE and ischemia-driven revascularization among patients with 3–4 SMuRFs. Therefore, screening for OSA should be emphasized in ACS patients with 3–4 SMuRFs, and intervention trials should be prioritized in these high-risk patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Standard modifiable risk factors (SMuRFs), including diabetes, hypertension, hyperlipidemia, and smoking, are routinely targeted strategies for prevention and treatment of cardiovascular disease and are associated with higher risk of cardiovascular events in patients with acute coronary syndrome (ACS) [1,2,3,4]. Obstructive sleep apnea (OSA), which accounts for 40–60% of patients with cardiovascular disease, is a potential modifiable risk factor for cardiovascular disease characterized by intermittent hypoxia, activation of sympathetic nervous system, and sleep fragmentation [5, 6]. Accumulating studies have elucidated that OSA is an independent risk factor for long-term prognosis of cardiovascular disease [7, 8]. Additionally, a growing body of evidence suggests a bidirectional relationship between OSA and SMuRFs. OSA is an independent risk factor for hypertension [9], diabetes [10], and hyperlipidemia [11], while patients with these factors are also a susceptible population for OSA [12]. However, the prevalence and prognostic implication of OSA in ACS patients with different amounts of SMuRFs remain unclear. Therefore, we aimed to elucidate the prognostic implication of OSA in ACS patients stratified by the number of SMuRFs.
Methods
Study design and population
This is an ancillary study of the OSA-ACS project (NCT03362385), which is a single-center, prospective, cohort study aimed at investigating the association between OSA and cardio-cerebrovascular events of ACS patients [13,14,15]. In brief, patients included in the project were hospitalized for ACS in Beijing Anzhen Hospital, Capital Medical University from January 2015 and December 2019, whose age was in a range of 18 to 85 years old. Patients with cardiogenic shock, cardiac arrest, or malignant tumor were excluded. A sleep study failing or lasting for less than 180 min was also considered as an exclusion criterion. Additionally, patients were also excluded who are lost to follow-up, with central sleep apnea, or with management of regular continuous positive airway pressure. The study followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines [16], complied with the Declaration of Helsinki and was approved by the local committee (2,013,025). Meanwhile, all patients signed informed consent.
Definition of SMuRFs
Standard modifiable risk factors include current smoking status, hypertension, hyperlipidemia, and diabetes [17]. Hypertension was defined as self-reported hypertension or using antihypertensive medication before admission; definition of hyperlipidemia referred to self-reported hyperlipidemia, using lipid-lowering medications before admission, an LDL-C concentration of 3.37 mmol/L or higher, or a total cholesterol concentration of 5.18 mmol/L; diabetes was defined as having a previous diagnosis of diabetes or previous glucose lowering pharmacotherapy. As both fasting glucose and acute phase blood pressure are influenced by neurohormonal response to admission status, these were not incorporated in the definitions.
Procedure and management
Portable cardiorespiratory polygraphy (Apnea-Link, ResMed, Australia) was applied to record the situation of arterial oxygen saturation, nasal airflow, snoring episodes, and thoracoabdominal movements in order to study nocturnal sleep-in stabilized patients. It was considered as a valid test when the polygraph recording was satisfactory and lasted at least 3 h. Airflow absence lasting ≥ 10 s was identified as apnea that was classified into obstructive apnea with thoracoabdominal movement and central apnea without thoracoabdominal movement. Hypopnea was defined as > 30% reduction in airflow for ≥ 10 s accompanied by a > 4% decrease in arterial oxygen saturation. Apnea-hypopnea index (AHI) refers to the cumulative number of apneas and hypopneas per hour during the total recording time. All parameters of sleep study were judged by two independent sleep technologists, while in cases of contradiction, further judgement was performed by a senior sleep medicine consultant. Recruited patients were stratified into OSA group (AHI ≥ 15 events per hour) and non-OSA group (AHI < 15 events per hour). All patients received guideline-directed medical therapy and if necessary, percutaneous coronary intervention or coronary artery bypass grafting was performed. Unless contraindicated, patients discharged from the hospital were administered dual antiplatelet therapy (aspirin plus clopidogrel or ticagrelor) for at least 1 year. Referral to sleep center was offered to patients with moderate to severe sleep apnea (AHI ≥ 15), especially those with excessive daytime sleepiness, in order to achieve further evaluation and intervention.
Follow-up and outcomes
After discharge, follow-up was performed at the clinic or via telephone at 1 month, 3 months, 6 months, 1 year, and then every 6 months. Major adverse cardiovascular and cerebrovascular events (MACCE) were the primary endpoints that included cardiovascular death, ischemia-driven revascularization, myocardial infarction, hospitalization for unstable angina or heart failure, and stroke. Secondary endpoints included components of MACCE, all repeat revascularization, a composite of cardiovascular death, myocardial infarction, or ischemic stroke, and a composite consisting of cardiac events which was the components of the primary end point after excluding stroke.
Statistical analysis
Quantitative data with normal distribution were described by mean ± standard deviation and compared via analysis of variance while non-normal distributed data were described by median (interquartile range) and the difference was analyzed via Kruskal-Walls test. The description of qualitative data was performed by frequency and percentage and the difference was discriminated by chi-square tests. Cox proportional hazards model was utilized to analyze the relationship between OSA and endpoint events after stratification of patients into subgroups according to the number of SMuRFs. Model covariates were determined based on clinical relevance or univariate relationships between variables and outcomes. Finally, we applied 3 models in the study: (1) unadjusted; (2) partially adjusted for age and sex; (3) fully adjusted for age (per 10-year increase), sex, body mass index, prior stroke, prior myocardial infarction, left ventricular ejection fraction (LVEF) less than 40%, systolic blood pressure (per 10 mm Hg increase), and plasma creatinine (per 10 µmol/L increase). Statistical analysis was performed via SPSS (version 26.0 IBM SPSS Inc, Armonk, NY) and a two-side P value less than 0.05 was the standard of significant difference.
Results
Baseline characteristics
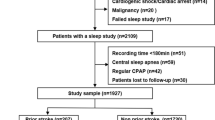
The project included 2016 ACS patients undergoing sleep study, of whom 2058 patients completed a sleep study and acquired valid results. After further screening based on exclusion criteria, we eventually enrolled 1927 patients for the final analysis (Fig. 1), among whom 130(6.7%) patients had no SMuRF, 1264(65.6%) patients had 1–2 SMuRFs and 533(27.7%) patients had 3–4 SMuRFs. The clinical characteristics of patients stratified by the number of SMuRFs was presented in Table 1. Patients with more SMuRFs were younger (P = 0.004), meanwhile, while with the accumulation of SMuRFs, there was an increase in body mass index (P < 0.001), waist to hip ratio (P < 0.001) and Hs-CRP (P = 0.03). Additionally, as the number of SMuRFs increased, patients with diabetes, hypertension, hyperlipidemia, and prior stroke accounted for a greater proportion and there were more current smokers and alcohol drinkers.
As depicted in Table 2 and Supplementary material, Figure S1, the proportion of patients without SMuRF combined with OSA was 47.7% and the proportion of patients with 1–2 SMuRF combined with OSA was 51.5%. Besides, the proportion of patients with 3–4 SMuRF combined with OSA was 56.6%. This presented a trend that the proportion of patients combined OSA increased with the increase of the number of SMURFs, but there was no statistical difference (P = 0.08). The median AHI was 14.5 (6.6–23.1), 15.6 (7.0-28.8) and 18.0 (8.5–36.2) events per hour among patients with no SMuRF, 1–2 SMuRFs, and 3–4 SMuRFs, respectively (P = 0.001). Compared with patients without SMuRFs, patients with more SMuRFs exhibited higher oxygen desaturation index (ODI) (P = 0.004) and longer time of arterial oxygen saturation less than 90% (P < 0.001), accompanied by a reduction of nadir arterial oxygen saturation(P < 0.001). Furthermore, patients with more SMuRFs were more probable to be administered the treatment of ACEIs/ARB (42.3% versus 60.0% versus 71.7, P < 0.001). Participants were also stratified by OSA to further describe their characteristics, as shown in Supplementary material, table S1 and table S2.
Effect of different number of risk factors on prognosis of ACS
The median of follow-up for the study was 34.97 (18.67–43.23) months. Kaplan-Meier analysis elucidated no difference in the cumulative incidence of MACCE for patients with different numbers of SMuRFs (Log-rank, P = 0.58; Supplementary material, Figure S2). Notably, during the late follow-up period, Kaplan-Meier analysis presented a growing trend towards cumulative incidence of MACCE with increasing number of SMuRFs. Data about crude number of all events in patients stratified by the number of SMuRFs were shown in Supplementary material, Table S3.
Outcomes of OSA versus non‑OSA patients stratified by number of SMuRFs
The correlation between OSA and risk of cardiovascular events was analyzed by cox regression stratified by number of SMuRFs. As depicted in Tables 3and Fig. 2, OSA in the 3–4 SMuRFs group had a significant influence on the incidence of MACCE (adjusted HR, 1.65; 95%CI, 1.06–2.57; P = 0.026) and ischemia-driven revascularization (adjusted HR, 2.18; 95%CI, 1.03–4.65; P = 0.042), after adjusted for age (per 10-year increase), sex, body mass index, prior stroke, prior myocardial infarction, left ventricular ejection fraction (LVEF) less than 40%, systolic blood pressure (per 10 mm Hg increase), and plasma creatinine (per 10 µmol/L increase). Data about crude number of all events in OSA or non-OSA patients overall or stratified by the number of SMuRFs were shown in Supplementary material, Table S4.
Cumulative Incidence of MACCE and Ischemia-driven Revascularization by SMuRFs and OSA Categories
Kaplan-Meier estimates and fully-adjusted HR for MACCE and Ischemia-driven Revascularization in patients with No SMuRF (A, D), 1-2SMuRFs (B, E), or 3–4 SMuRFs (C, F). Adjusted for age (per 10-year increase), sex, body mass index, prior stroke, prior myocardial infarction, left ventricular ejection fraction (LVEF) less than 40%, systolic blood pressure (per 10 mm Hg increase), plasma creatinine (per 10 µmol/L increase). MACCE, major adverse cardiovascular and cerebrovascular events; OSA, obstructive sleep apnea; SMuRFs, standard modifiable risk factors;
Discussion
In the study, we found that with the accumulation of SMuRFs, the symptom of OSA presented a more frequent incidence of apnea and hypopnea and a reduced oxygen saturation with a longer duration, which elucidated that patient with more SMuRFs had a worse situation of OSA. In hospitalized ACS patients, OSA is associated with an increased risk of MACCE and ischemia-driven revascularization among patients with 3–4 SMuRFs.
Hypoxia evoked by OSA in ACS patients with SMuRFs
Our study founded that the proportion of OSA in ACS patients exhibited an increased trend with the accumulation of SMuRFs (47.7% versus 51.5% versus 56.6%), but there was no significant difference (P = 0.08). Nonetheless, the higher AHI and ODI, the longer time of arterial oxygen saturation less than 90%, and the reduction of nadir arterial oxygen saturation elucidated the potential worsening hypoxia evoked by OSA in ACS patients with more SMuRFs. Hypoxia during sleep is one of the distinguished characteristics of OSA pathophysiological process [18]. The repetitive process of desaturation and reoxygenation named intermittent hypoxia in patients with OSA activated the hypoxia-sensitive transcription factor-1 and NF-kB [19]. After the activation, adhesion factors are up-regulated, eliciting the recruitment and migration of macrophage, facilitating the inflammation process [20]. Eventually, hypoxia caused by OSA contributes to coronary atherosclerosis and vascular events [21, 22]. Therefore, given that the exacerbation of hypoxia caused by OSA with the accumulation of SMuRFs, we hypothesized that OSA might insult an increased incidence of cardio- cerebrovascular events in ACS patients with a greater number of SMuRFs.
OSA as a synergistic risk factor in ACS patients with 3–4 SMuRFs
It is acknowledged that the accumulation of SMuRFs means an increased burden that is implicated with a greater risk of cardiovascular events [23]. In contrast, some previous research held a different view that the absence of SMuRFs contributed to a higher mortality in patients with acute coronary syndrome [24,25,26]. Figtree et al. argued that fewer application of drugs improving prognosis, including ACEI/ARB, β-blocker, and statin, accounted for the controversy, after adjusting for which their results elucidated that patient with more SMuRFs conferred an increased risk of mortality in patients with segment elevated myocardial infarction [27]. Patients without SMuRFs had lower odds of all cause and cardiac mortality and major adverse cardiovascular events after propensity score matching [17].
Although there exists no study on the association between OSA and SMuRFs, previous literatures have probed the impact of OSA on clinical outcomes in patients with heart disease complicated by diabetes, hypertension, or hyperlipidemia [28]. The severity of OSA is an independent risk factor for insulin resistance via the activation of the hypothalamus-adrenal axis, increasing the cardiovascular disease risk [29]. Previous studies have also demonstrated that diabetes patients with OSA had a higher proportion of microvascular complications and increased the risk of MACE, unstable angina hospitalization, and all-cause mortality [30,31,32]. Therefore, OSA is potentially a synergistic risk factor for diabetes [33]. Hypoxia induced by OSA promotes systemic inflammation and oxidative stress, which results in the upregulation of endothelin-1 and decrease of nitric oxide in endothelial cells, insulting increased arterial peripheral resistance and elevation of blood pressure; besides, OSA also affects elevation of blood pressure via activating sympathetic nerve. Eventually, OSA accelerates the adverse cardiovascular remodeling in patients with resistant hypertension [34]. OSA also contributes to dyslipidemia via upregulating involved enzymes to induce the increase of liver lipid synthesis [35].
In our study, OSA was in correlation with an increased incidence of MACCE and ischemia-driven revascularization events in ACS patients with 3–4 SMuRFs after adjusting for confounding factors and hence we think OSA serves as a synergistic risk factor in ACS patients with 3–4 SMuRFs and it is necessary to screen for OSA in ACS patients, especially in whom have more SMuRFs.
Intervention of OSA in ACS patients with SMuRFs
Although OSA is an independent risk factor for cardiovascular events in ACS patients, the effect of continuous positive airway pressure (CPAP) on secondary cardiovascular protection is controversial. Previous literature elucidated that CPAP showed no reduction in the incidence of cardiovascular events [36], while Fuji Yoshi et al. showed that CPAP reduces the incidence of cardiovascular events and the accumulation of macrophage in culprit lesions [37]. The difference might be attributed to the phenotype of OSA, for instance, the phenotype of OSA in patients with coronary artery disease and diabetes is at high risk of cardiovascular events and benefits from the treatment of CPAP [38]. Given the synergistic effect of OSA in ACS patients with SMuRFs and the reduced influence of OSA intervention on cardiovascular risk factors, such as blood pressure [39] and blood glucose [40], we recommend that OSA in ACS patients with SMuRFs should receive the treatment of CPAP. The lack of relevant researches prompt further research to explore the benefits of CPAP for the ACS population with SMURFs.
Limitations
There existed some limitations in this study. First, we conducted this study in ACS patients while OSA severity was potentially overestimated in any high-risk disease setting. Second, we assessed the OSA with the portable polygraphy which possibly underestimated the severity caused by overestimation of actual sleeping time. Third, this study is based on a prospective cohort study, which inevitably affects the results due to its own disadvantages. Finally, this study was an observational, single-center study recruiting patients from China, which made it necessary to further validate the conclusion in cohort studies and limited the extension to more ethic population.
Conclusions
In hospitalized ACS patients, OSA is associated with an increased risk of MACCE and ischemia-driven revascularization among patients with 3–4 SMuRFs. Therefore, screening for OSA should be emphasized in ACS patients with 3–4 SMuRFs, and intervention trials should be prioritized in these high-risk patients.
Data availability
Data collected and analyzed in this study are included in this article or the supplementary material files.
References
Besnier M, Finemore M, Yu C, Kott KA, Vernon ST, Seebacher NA, Genetzakis E, Furman A, Tang O, Davis RL, Hansen T, Psaltis PJ, Bubb KJ, Wise SG, Grieve SM, Di Bartolo BA, Figtree GA (2021) Patient Endothelial Colony-Forming Cells to Model Coronary Artery Disease Susceptibility and Unravel the Role of Dysregulated Mitochondrial Redox Signalling. Antioxidants (Basel, Switzerland) 10. https://doi.org/10.3390/antiox10101547
Stomby A, Strömberg S, Theodorsson E, Olsen Faresjö Ã, Jones M, Faresjö T (2021) Standard Modifiable Cardiovascular risk factors mediate the Association between elevated hair cortisol concentrations and coronary artery disease. Front Cardiovasc Med 8:765000. https://doi.org/10.3389/fcvm.2021.765000
Wu Y, Zhang GY, Hu R, Du JL (2021) Risk of Target Organ damage in patients with masked hypertension versus sustained hypertension: a Meta-analysis. Cardiovasc Innovations Appl 5:155–163. https://doi.org/10.15212/cvia.2019.1261
Sokhal BS, Matetić A, Paul TK, Velagapudi P, Lambrinou E, Figtree GA, Rashid M, Moledina S, Vassiliou VS, Mallen C, Mamas MA (2023) Management and outcomes of patients admitted with type 2 myocardial infarction with and without standard modifiable risk factors. Int J Cardiol 371:391–396. https://doi.org/10.1016/j.ijcard.2022.09.037
Tietjens JR, Claman D, Kezirian EJ, De Marco T, Mirzayan A, Sadroonri B, Goldberg AN, Long C, Gerstenfeld EP, Yeghiazarians Y (2019) Obstructive sleep apnea in Cardiovascular Disease: a review of the literature and proposed Multidisciplinary Clinical Management Strategy. J Am Heart Assoc 8:e010440. https://doi.org/10.1161/jaha.118.010440
Naito R, Kasai T, Dohi T, Takaya H, Narui K, Momomura SI (2022) Factors Associated with the improvement of left ventricular systolic function by continuous positive Airway pressure therapy in patients with heart failure with reduced ejection Fraction and Obstructive Sleep Apnea. Front Neurol 13:781054. https://doi.org/10.3389/fneur.2022.781054
Li JH, Gao YH, Xue X, Su XF, Wang HH, Lin JL, Zhao LB, Zou X, Gao Y, Guo JJ, Shi M, Xu WH, Wang YB, Qian XS, Chen KB, Fan L, Liu L (2022) Association between serum cystatin C levels and long-term cardiovascular outcomes and all-cause mortality in older patients with obstructive sleep apnea. Front Physiol 13:934413. https://doi.org/10.3389/fphys.2022.934413
Harańczyk M, Konieczyńska M, Płazak W (2022) Endothelial dysfunction in obstructive sleep apnea patients. Sleep & breathing = Schlaf & Atmung 26:231–242. https://doi.org/10.1007/s11325-021-02382-4
Kario K, Hettrick DA, Prejbisz A, Januszewicz A (2021) Obstructive Sleep Apnea-Induced Neurogenic Nocturnal Hypertension: A Potential Role of Renal Denervation? Hypertension (Dallas, Tex: 1979) 77: 1047-60. https://doi.org/10.1161/hypertensionaha.120.16378
Huang T, Lin BM, Stampfer MJ, Tworoger SS, Hu FB, Redline S (2018) A Population-Based study of the Bidirectional Association between Obstructive Sleep Apnea and Type 2 diabetes in three prospective U.S. cohorts. Diabetes Care 41:2111–2119. https://doi.org/10.2337/dc18-0675
Martínez-Cerón E, Casitas R, Galera R, Sánchez-Sánchez B, Zamarrón E, Garcia-Sanchez A, Jaureguizar A, Cubillos-Zapata C, Garcia-Rio F (2021) Contribution of sleep characteristics to the association between obstructive sleep apnea and dyslipidemia. Sleep Med 84:63–72. https://doi.org/10.1016/j.sleep.2021.05.012
Gleeson M, McNicholas WT (2022) Bidirectional relationships of comorbidity with obstructive sleep apnoea. Eur respiratory review: official J Eur Respiratory Soc 31. https://doi.org/10.1183/16000617.0256-2021
Wang X, Fan J, Guo R, Hao W, Gong W, Yan Y, Zheng W, Ai H, Que B, Hu D, Ma C, Ma X, Somers VK, Nie S (2023) Association of obstructive sleep apnoea with cardiovascular events in women and men with acute coronary syndrome. Eur Respir J 61. https://doi.org/10.1183/13993003.01110-2022
Fan JY, Wang X, Ma XL, Somers VK, Nie SP, Wei YX (2019) Association of Obstructive Sleep Apnea with Cardiovascular Outcomes in patients with Acute Coronary Syndrome. J Am Heart Association 8. https://doi.org/10.1161/jaha.118.010826
Hao W, Wang X, Fan J, Guo R, Gong W, Yan Y, Zheng W, Que B, Ai H, Ma C, Ma X, Nie S (2023) Prognostic implications of OSA in Acute Coronary syndrome by obesity status. Chest. https://doi.org/10.1016/j.chest.2023.02.001
Cuschieri S (2019) The STROBE guidelines. Saudi J Anaesth 13:31–34. https://doi.org/10.4103/sja.SJA_543_18
Moledina SM, Rashid M, Nolan J, Nakao K, Sun LY, Velagapudi P, Wilton SB, Volgman AS, Gale CP, Mamas MA (2022) Addressing disparities of care in non-ST-segment elevation myocardial infarction patients without standard modifiable risk factors: insights from a nationwide cohort study. Eur J Prev Cardiol 29:1084–1092. https://doi.org/10.1093/eurjpc/zwab200
Vaccarino V, Shah AJ, Moncayo V, Nye JA, Piccinelli M, Ko YA, Ma X, Murrah N, Shallenberger L, Driggers E, Jajeh N, Haffar A, Al-Abboud O, Raggi P, Hall MH, Sloan RP, Goldberg J, Smith NL, Garcia EV, Quyyumi AA, Bremner JD, Bliwise DL (2022) Obstructive sleep apnea, myocardial perfusion and myocardial blood flow: a study of older male twins. PLoS ONE 17:e0278420. https://doi.org/10.1371/journal.pone.0278420
Li YE, Ren J (2022) Association between obstructive sleep apnea and cardiovascular diseases. Acta Biochim Biophys Sin 54:882–892. https://doi.org/10.3724/abbs.2022084
Ooi EL, Rajendran S (2022) Obstructive sleep apnea in coronary artery disease. Curr Probl Cardiol 101178. https://doi.org/10.1016/j.cpcardiol.2022.101178
Goodman MO, Cade BE, Shah NA, Huang T, Dashti HS, Saxena R, Rutter MK, Libby P, Sofer T, Redline S (2022) Pathway-specific polygenic risk scores identify obstructive sleep apnea-related pathways differentially moderating genetic susceptibility to coronary artery disease. Circulation Genomic and precision medicine 15:e003535. https://doi.org/10.1161/circgen.121.003535
Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N, Mehra R, Bozkurt B, Ndumele CE, Somers VK (2021) Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 144:e56–e67. https://doi.org/10.1161/cir.0000000000000988
Li S, Gao X, Yang J, Xu H, Wang Y, Zhao Y, Yin L, Wu C, Wang Y, Zheng Y, Li B, Zhang X, Ye Y, Fu R, Dong Q, Sun H, Yan X, Wu Y, Zhang J, Jin C, Li W, Yang Y (2022) Number of standard modifiable risk factors and mortality in patients with first-presentation ST-segment elevation myocardial infarction: insights from China Acute myocardial infarction registry. BMC Med 20:217. https://doi.org/10.1186/s12916-022-02418-w
Kong G, Chew NWS, Ng CH, Chin YH, Zeng R, Foo R, Chan KH, Low AF, Lee CH, Chan MY, Yeo TC, Tan HC, Loh PH (2022) Long-term outcomes in acute coronary syndrome patients without standard modifiable risk factors: a multi-ethnic retrospective cohort study of 5400 asian patients. J Thromb Thrombolysis 54:569–578. https://doi.org/10.1007/s11239-022-02704-7
Papazoglou AS, Farmakis IT, Zafeiropoulos S, Moysidis DV, Karagiannidis E, Stalikas N, Kartas A, Stamos K, Sofidis G, Doundoulakis I, Giannopoulos G, Giannakoulas G, Sianos G (2022) Angiographic severity in acute coronary syndrome patients with and without standard modifiable risk factors. Front Cardiovasc Med 9:934946. https://doi.org/10.3389/fcvm.2022.934946
Sia CH, Ko J, Zheng H, Ho AF, Foo D, Foo LL, Lim PZ, Liew BW, Chai P, Yeo TC, Yip JWL, Chua T, Chan MY, Tan JWC, Figtree G, Bulluck H, Hausenloy DJ (2022) Comparison of mortality outcomes in Acute myocardial infarction patients with or without Standard Modifiable Cardiovascular Risk factors. Front Cardiovasc Med 9:876465. https://doi.org/10.3389/fcvm.2022.876465
Figtree GA, Vernon ST, Hadziosmanovic N, Sundström J, Alfredsson J, Arnott C, Delatour V, Leósdóttir M, Hagström E (2021) Mortality in STEMI patients without standard modifiable risk factors: a sex-disaggregated analysis of SWEDEHEART registry data. Lancet (London England) 397:1085–1094. https://doi.org/10.1016/s0140-6736(21)00272-5
Xu Y, Ye Z, Wang B, Tang L, Sun J, Chen X, Yang Y, Wang J (2022) Novel Insights into the Predictors of Obstructive Sleep Apnea Syndrome in Patients with Chronic Coronary Syndrome: Development of a Predicting Model. Oxidative medicine and cellular longevity 2022: 5497134. https://doi.org/10.1155/2022/5497134
Bouloukaki I, Schiza S, Tsiligianni I (2021) Obstructive sleep apnea as an additive or even synergistic risk factor for cardiovascular disease in patients with type 2 diabetes: a call for action in primary care? Diabetes Res Clin Pract 178:108940. https://doi.org/10.1016/j.diabres.2021.108940
Su X, Li JH, Gao Y, Chen K, Gao Y, Guo JJ, Shi M, Zou X, Xu W, Zhao LB, Wang H, Wang Y, Liu J, Xu H, Kong X, Lin J, Qian X, Han J, Liu L (2021) Impact of obstructive sleep apnea complicated with type 2 diabetes on long-term cardiovascular risks and all-cause mortality in elderly patients. BMC Geriatr 21:508. https://doi.org/10.1186/s12877-021-02461-x
Wang H, Li X, Tang Z, Gong G (2020) Cardiovascular Outcomes Post Percutaneous Coronary intervention in patients with obstructive sleep apnea and type 2 diabetes Mellitus: a systematic review and Meta-analysis. Diabetes therapy: research treatment and education of diabetes and related disorders 11:1795–1806. https://doi.org/10.1007/s13300-020-00870-6
Protasiewicz Timofticiuc DC, Vladu IM, Ștefan AG, Clenciu D, Mitrea A, Pădureanu V, Efrem IC, Diaconu ID, Turcu A, Țenea-Cojan T, Hâncu AM, Forțofoiu M, Mirea Munteanu O, Moța M (2022) Associations of Chronic Diabetes Complications and Cardiovascular Risk with the risk of obstructive sleep apnea in patients with type 2 diabetes. J Clin Med 11. https://doi.org/10.3390/jcm11154403
Wang X, Fan J, Du Y, Ma C, Ma X, Nie S, Wei Y (2019) Clinical significance of obstructive sleep apnea in patients with acute coronary syndrome in relation to diabetes status. BMJ open diabetes research & care 7:e000737. https://doi.org/10.1136/bmjdrc-2019-000737
Salman LA, Shulman R, Cohen JB (2020) Obstructive sleep apnea, hypertension, and Cardiovascular Risk: Epidemiology, Pathophysiology, and management. Curr Cardiol Rep 22:6. https://doi.org/10.1007/s11886-020-1257-y
Zeljković A, Milojević A, Vladimirov S, Zdravković M, Memon L, Brajković M, Gardijan V, Gojković T, Stefanović A, Miljković-Trailović M, Spasojević-Kalimanovska V, Ninić A (2022) Alterations of cholesterol synthesis and absorption in obstructive sleep apnea: influence of obesity and disease severity. Nutr metabolism Cardiovasc diseases: NMCD 32:2848–2857. https://doi.org/10.1016/j.numecd.2022.09.006
Sánchez-de-la-Torre M, Sánchez-de-la-Torre A, Bertran S, Abad J, Duran-Cantolla J, Cabriada V, Mediano O, Masdeu MJ, Alonso ML, Masa JF, Barceló A, de la Peña M, Mayos M, Coloma R, Montserrat JM, Chiner E, Perelló S, Rubinós G, Mínguez O, Pascual L, Cortijo A, Martínez D, Aldomà A, Dalmases M, McEvoy RD, Barbé F (2020) Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. The Lancet Respiratory medicine 8:359–367. https://doi.org/10.1016/s2213-2600(19)30271-1
Fujiyoshi K, Tojo T, Minami Y, Ishida K, Ishida M, Wakabayashi KI, Inomata T, Ako J (2023) Clinical outcomes and plaque characteristics in patients with coronary artery disease and concomitant sleep-disordered breathing treated by continuous positive airway pressure. Sleep Med 101:543–549. https://doi.org/10.1016/j.sleep.2022.12.014
Quan W, Zheng D, Douglas McEvoy R, Barbe F, Chen R, Liu Z, Loffler K, Lorenzi-Filho G, Luo Y, Mukherjee S, Tripathi M, Woodman R, Li Q, Wang X, Arima H, Xiao Y, Zhang X, Anderson CS (2018) High Risk Characteristics for Recurrent Cardiovascular Events among Patients with Obstructive Sleep Apnoea in the SAVE Study. EClinicalMedicine 2–3: 59–65. https://doi.org/10.1016/j.eclinm.2018.09.002
Wang X, Guan L, Wu C, Zhao Y, Zhao G (2023) Continuous positive airway pressure may improve hypertension in patients with obstructive sleep apnea-hypopnea syndrome by inhibiting inflammation and oxidative stress. Archives of medical science: AMS 19:237–241. https://doi.org/10.5114/aoms/156490
O’Connor-Reina C, Alcala LR, Ignacio JM, Iriarte MTG, Llatas MC, Morente JCC, Del Rey DP, Alvarez IM, Ibarburu GH, Baptista P, Plaza G (2023) Risk of diabetes in patients with sleep apnea: comparison of surgery versus CPAP in a long-term follow-up study. Journal of otolaryngology - head & neck surgery = Le Journal d’oto-rhino-laryngologie et de chirurgie cervico-faciale 52: 16. https://doi.org/10.1186/s40463-022-00616-3
Funding
This study was supported by Beijing Nova Program (Z201100006820087), Interdisciplinary Cooperation Project of Beijing Nova Program (Z211100002121165), Natural Science Foundation of Beijing, China (7222046, 7191002), National Key Research & Development Program of China (2022YFC2505600, 2020YFC2004800), National Natural Science Foundation of China (82270258, 82200495).
Author information
Authors and Affiliations
Contributions
Bin Wang and Yuekun Zhang completed the preliminary data analysis and manuscript writing; Wen Hao and Jingyao Fan finished the collation and verification of sleep related data; Hui Ai, Bin Que and Yan Yan completed patient enrollment and clinical information collection; Wei Gong and Wen Zheng finished collecting all the data; Xiao Wang and Shaoping Nie finished the revision of the manuscript.
Corresponding authors
Ethics declarations
Competing of Interests
All authors have no competing interests to declare that are relevant to the content of this article.
Ethics approval
This study complied with the Declaration of Helsinki and was approved by the local committee (2013025).
Informed consent
All patients signed informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, B., Zhang, Y., Hao, W. et al. Effect of obstructive sleep apnea on prognosis in patients with acute coronary syndromes with varying numbers of standard modifiable risk factors: insight from the OSA-ACS study. J Thromb Thrombolysis 56, 65–74 (2023). https://doi.org/10.1007/s11239-023-02830-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-023-02830-w