Abstract
The optimal management strategy for submassive or intermediate risk pulmonary embolism (IRPE)—anticoagulation alone versus anticoagulation plus advanced therapies—remains in equipoise leading many institutions to create multidisciplinary PE response teams (PERTs) to guide therapy. Cause-specific mortality of IRPE has not been thoroughly examined, which is a meaningful outcome when examining the effect of specific interventions for PE. In this retrospective study, we reviewed all adult inpatient admissions between 8/1/2018 and 8/1/2019 with an encounter diagnosis of PE to study all cause and PE cause specific mortality as the primary outcomes and bleeding complications from therapies as a secondary outcome. There were 429 total inpatient admissions, of which 59.7% were IRPE. The IRPE 30-day all-cause mortality was 8.7% and PE cause-specific mortality was 0.79%. Treatment consisted of anticoagulation alone in 93.4% of cases. Advanced therapies—systemic thrombolysis, catheter directed thrombolysis, or mechanical thrombectomy, were performed in only six IRPE cases (2.3%). Decompensation of IRPE cases requiring higher level of care and/or rescue advanced therapy occurred in only five cases (2%). In-hospital major bleeding and clinically relevant non-major bleeding were more common in those receiving systemic thrombolysis (61.5%) compared to anticoagulation combined with other advanced therapies (11.7%). Despite the high overall acuity of PE cases at our institution, in-hospital all-cause mortality was low and cause-specific mortality for IRPE was rare. These data suggest the need to target other clinically meaningful outcomes when examining advanced therapies for IRPE.
Similar content being viewed by others
Highlights
-
Cause-specific mortality in acute intermediate risk pulmonary embolism is very low.
-
Mortality in patients with PE is mostly from their underlying illness, such as malignancy, rather than PE.
-
Advanced interventional therapies were rarely used in this cohort for IRPE. Future directions: There is a need to study outcomes appropriate to PE when studying interventions in clinical research instead of generalized mortality.
Introduction
Pulmonary embolism (PE) is a common and potentially fatal condition with an estimated incidence of a 112 to 115 per 100,000 adults in the United States [1,2,3]. Several treatment options are now available to treat PE. While there is broad consensus regarding the management of low and high risk PE, controversy persists regarding IRPE, in terms of both preventing short term mortality and long-term sequelae [4,5,6]. Fibrinolysis in IRPE has been found to decrease the risk of the composite outcome of death or hemodynamic compromise at the expense of increased risk of stroke and major hemorrhage, though some of that risk can be mitigated by using lower doses of thrombolytics [7, 8]. Long term follow-up of fibrinolytic therapy in IRPE has not demonstrated a difference in mortality or the prevalence of pulmonary hypertension [9]. A systematic review including 18 randomized trials examining thrombolytic therapy for both hemodynamically stable and unstable PE showed a reduction in death with thrombolytic therapy, but this effect was lost when studies with a high risk of bias were excluded [10]. Another meta-analysis found that compared to standard anticoagulation, recanalization procedures including systemic thrombolysis and catheter directed thrombolysis had similar risk of all-cause mortality when compared with standard anticoagulation [11]. In the last decade, several institutions have implemented multidisciplinary Pulmonary Embolism Response Teams (PERT) to rapidly evaluate intermediate and high risk PE cases and formulate individualized treatment plans [12, 13]. At least one institution has reported a reduction in all-cause mortality compared to the year prior to adopting a PERT [14], though PE-specific mortality was not examined. Determining PE cause specific mortality, and rates of hemodynamic decompensation would allow for better determination of the impact of PE-specific treatments, especially in the IRPE group [15].
To better define the all-cause and PE cause-specific mortality rates, a retrospective cohort of consecutive patients hospitalized (8/1/2018–8/1/2019) with PE was analyzed. We further sought to assess the impact of patient characteristics and treatment on clinical outcomes for these patients.
Methods
A single-center retrospective observational study was conducted at the Mayo Clinic in Rochester, MN where PE cases are treated without the use of a PERT. The study protocol was reviewed by the Institutional Review Board and deemed it exempt from the requirement of IRB approval (45 CFR 46.104d, category 4). The primary outcome was to ascertain both all-cause and PE cause-specific mortality in all PE cases managed in the inpatient setting. Additionally, we examined and described in detail the clinical characteristics and treatment strategies used to elucidate important variables leading to mortality or hemodynamic decompensation. The secondary outcome was to study the bleeding complications from therapies.
All adult (age > 18) inpatient admissions at two inpatient facilities of the Mayo Clinic in Rochester, MN between 8/1/2018 and 8/1/2019 with an encounter diagnosis of pulmonary embolism (ICD-9 415.1; ICD-10 I26) diagnosed via computed tomography angiogram (CTA) or ventilation-perfusion scans were reviewed. All consecutive cases were included, and patient charts were individually reviewed by HM and SA. Demographic, clinical, radiological, laboratory, and treatment data were collected from which the Pulmonary Embolism Severity Index (PESI) score was calculated [16]. In order to better understand the entire spectrum of disease presentation for hospitalized patients with PE, no patients were excluded from the analysis.
The cases were stratified into low, intermediate, and high risk according to the ACCP guidelines [5, 17]. Briefly, a PE was considered intermediate risk (also referred to as submassive or moderate) if either right ventricular dysfunction or elevation of biomarkers (troponin or brain natriuretic peptide) was present. High risk PE were those associated with systolic blood pressure < 90 mm Hg for at least 15 min. All others were low risk PE. The presence of right ventricular dysfunction was ascertained from the radiologists’ report of the diagnostic CT scan or from the echocardiogram report if one was performed within 24 h of PE diagnosis. Serum biomarker levels of N-terminal pro B-Type Natriuretic Peptide (NT ProBNP considered abnormal if > 300 pg/mL) and fifth generation troponin T (abnormal if > 15 ng/L for males and > 10 ng/L for females) were noted. Cases with evidence of cancer (either untreated or undergoing active treatment) at the time of PE diagnosis were categorized as having active malignancy.
Therapeutic interventions were noted including initial anticoagulant used, and the usage of any advanced therapies/interventions. These included systemic (i.e., intravenous) thrombolytics with recombinant tissue plasminogen activator (rTPA), catheter directed thrombolysis, mechanical thrombectomy (performed by interventional radiology at our institution), extracorporeal membrane oxygenation (ECMO), and inferior vena cava (IVC) filter placement. The patient records were also reviewed for evidence of bleeding. Major bleeding and clinically relevant non-major (CRNM) bleeding were defined as previously described by the International Society of Thrombosis and Hemostasis (ISTH) [18, 19]. Major bleeding includes fatal bleeding, bleeding in a critical organ, fall in hemoglobin of 2 g/dL and need for transfusion of 2 units of packed red blood cells. CRNM is minor bleeding that still needs to be reported as a patient- centered outcome.
We defined mortality to be PE cause specific if determined by autopsy (when available) or if determined by the treating clinician to be definitely or probably due to PE as documented in the medical record. In-hospital, 30-day, 3-month, and 6-month mortality rates were examined. The total number of individual patients, rather than number of admissions, was used as the denominator to calculate mortality. In patients who survived the initial hospitalization to discharge, their setting of death (another inpatient admission, outpatient, or hospice) was noted. All statistical analysis was performed using BlueSky Statistics software. Differences among the 3 PE groups were analyzed using one way ANOVA and chi-square 3-sample test for equality of proportions.
Results
Over the one-year review period, 429 separate admissions with a diagnosis of PE were identified. Four (0.9%) of these patients had more than one admission for PE (confirmed by CTA each time); three patients had one readmission each, and one patient had two readmissions. Three patients were anticoagulated during their initial admission. One patient was treated with supportive care and IVC filter due to active alveolar hemorrhage. Another patient’s anticoagulation was stopped as an outpatient prior to readmission due to retroperitoneal bleeding (see Supplemental Table 1). Of these, one patient died during the study period for whom the time to death was counted from the initial PE event.
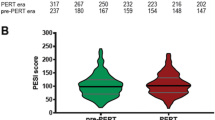
Patient demographics and clinical characteristics are provided in Table 1. Of the 429 admissions, 10 were high-risk (massive) PE, 256 were IRPE (submassive) and the remaining were low risk. Patients in the IRPE group were older than the other groups. As expected, PESI scores were significantly different between groups. Within each group, the range of PESI scores was broad, reflecting significant intra-group heterogeneity. There was a high prevalence of any history of malignancy (N = 189, 44%) and active malignancy (N = 158, 36.8%) in the entire cohort, but no statistically significant difference was present in the rates of active malignancy between the three groups. Provoking factors for PE included recent inpatient hospitalization (N = 90, 20.9%), recent surgery (N = 85, 19.8%), medications (N = 29, 6.7%), primary thrombophilia disorders (N = 28, 6.5%), trauma (N = 16, 3.7%) and underlying non-infectious inflammatory disorders (N = 8, 1.9%). A previous history of venous thromboembolism (DVT or PE) was present in 111 (25.8%) cases. In 68 (15.8%) cases, there was no provocative cause identified. Of the 331 patients who had a Doppler ultrasound of the extremities, 209 (63.1%) were positive for DVT. A significantly higher percentage of the intermediate-risk subgroup was positive for DVT compared to the low-risk subgroup—68.8% vs. 55.3%, respectively.
Mortality data is provided in Table 2. In the entire cohort, there were 11 in-hospital deaths, resulting in an all cause in-hospital mortality of 2.6%. PE cause-specific in-hospital mortality included two deaths in the high-risk group (20% of high-risk PE patients) and one death in the IRPE group (0.4% of IRPE patients). In the IRPE group, PE cause-specific mortality rate both in-hospital and for the 6-month study period was very low at 0.4% (N = 1) and 1.6% (N = 4), respectively. Cancer related deaths were a major factor in overall mortality. By the 30-day time point, 73% (N = 16) of deaths in the IRPE group and 100% of deaths (N = 7) in the low-risk group were patients with active cancer at the time. A significant portion of the patients who died enrolled in hospice care while hemodynamically stable, either as outpatients or during subsequent unrelated admissions. Of the eight patients (80%) surviving their initial hospitalization for high-risk PE, all were alive at the six-month mark. Of the 11 in-hospital deaths, autopsies were performed on two patients. For the entire 6-month duration of the study, autopsies were available for 11.4% (N = 10) of the deaths.
Hemodynamic decompensation after admission is a significant outcome in IRPE. In our study, five patients (2%) with IRPE at admission, then treated initially with anticoagulation alone, had subsequent decompensation requiring escalation of care (Table 3). Four of the five patients suffered obstructive shock but were successfully rescued with systemic alteplase. One patient suffered a sudden cardiac arrest with an initial rhythm of pulseless electrical activity. This patient did not receive systemic thrombolytics or other advanced therapies and did not survive the episode; this was classified as a PE cause-specific death.
Table 4 details the management strategies used. Just over 1% (N = 5) were treated with supportive care only whose clinical details are provided in Supplementary Table 2. The first anticoagulant used in most cases was unfractionated heparin. The direct oral anticoagulants (apixaban, rivaroxaban etc.) were rarely used as the first anticoagulant of choice—4.3% (N = 7) in the low-risk group and 3.0% (N = 8) in the IRPE group. A minority of patients underwent any of the advanced interventions described earlier. Only three patients underwent either catheter directed thrombolysis or mechanical thrombectomy. No patients underwent surgical thrombectomy. Excluding IVC filters, only six patients (2.3%) in the IRPE group underwent advanced therapies of any kind—one patient underwent catheter directed thrombolysis and another underwent thrombectomy (both performed by interventional radiology) due to marked hypoxemia persisting to day 3 and day 5 of hospitalization, respectively. IVC filter placement occurred in 23 cases (5.4%) among the entire cohort and in 9 cases (3.9%) in the IRPE group. Most patients getting IVC filters did so because of contraindications to anticoagulation or due to planned interruption in anticoagulation (see Supplemental Table 2).
In-hospital bleeding complications are detailed in Table 5. Overall incidence of bleeding (including major and CRNM bleeding) was low at 13% and even lower (11.7%) in those not receiving systemic thrombolysis. The subgroup of patients who received systemic thrombolysis had a much higher incidence of bleeding; this group also included three patients managed on VA ECMO, which is known to carry a high risk of bleeding independent of thrombolytics [20]. Only two patients underwent catheter-based interventions (one mechanical thrombectomy and another catheter directed thrombolysis) and neither had bleeding complications. Intracranial bleeding occurred in two patients, both of whom received intravenous alteplase; neither of them received ECMO support. One of those patients had a subarachnoid hemorrhage and another had a subdural hemorrhage. The two cases with significant vascular access site bleeding were both on ECMO support and had significant bleeding from the ECMO cannulation sites.
Discussion
Our cohort of inpatients is defined by a high risk PESI score, a large proportion of patients with malignancy, and a majority classified as intermediate risk PE. Overall, in-hospital PE-specific mortality was 0.7% and in-hospital IRPE-specific mortality 0.4%. Management was limited to anticoagulation alone in most patients without hemodynamic instability, and invasive procedures targeting the pulmonary vasculature were rarely performed. Four of five patients in the intermediate risk group that decompensated were successfully rescued. One patient with pancreatic cancer had sudden death. Serious bleeding in the context of relatively conservative management was uncommon.
Intermediate risk PE presents many challenges when considering management strategies and patient selection for advanced therapies. Direct mortality related to intermediate risk PE remains incompletely understood. A composite outcome of death or clinical decompensation is often used in clinical research [21]. Our study adds to the field by examining cause-specific mortality attributable to PE at a high volume tertiary care center in patients receiving the current standard of care. The overall acuity of PE cases at our institution was high with a mean PESI score of 112, which falls in the class IV (high risk) category. Our overall 30-day mortality rate of 7.3% is congruent with the expected rate predicted by that PESI score [16, 22]. Cause-specific mortality from PE in-hospital and at 30 days was less than 1% in our cohort. This low rate is unlikely to be significantly affected by any specific intervention or at least would require a very large population in a prospective study to demonstrate efficacy. In this cohort, the majority of patient deaths were related to their underlying malignancy, with a large portion transitioning to hospice care while hemodynamically stable. The rate of clinical decompensation was found to be only 1.9% in the intermediate risk group. Incidence of major bleeding was low in patients managed with anticoagulation alone, but higher in the group receiving systemic thrombolytics plus anticoagulation. Unfractionated heparin was used in most cases, including in the low-risk group. We speculate that the low rate of direct oral anticoagulant use may have been due to the high rate of comorbidities in this cohort and potential need for procedures necessitating interruption of anticoagulation during hospitalization, though the study was not designed to ascertain the rationale behind the clinicians’ choice of anticoagulation.
Whereas a conservative strategy of using anticoagulation alone may delay needed intervention in patients at risk of decompensation, a strategy employing advanced interventions widely across the IRPE group can place these patients at a higher bleeding risk. Given the low cause-specific mortality and low rate of decompensation in the IRPE group, a conservative therapeutic strategy in this subgroup with anticoagulation alone is reasonable as it avoids exposing patients to the potential adverse effects of more aggressive interventions. Several institutions have formed PERTs over the last decade to bring together the expertise of a multidisciplinary team to aid in complex clinical decision making and management of difficult cases. At the Mayo Clinic in Rochester, MN, we do not have a formal PERT. We do, however, have a strong culture of multi-disciplinary collaboration in patient care, especially in complex scenarios. Published experiences from institutions with PERTs do report an increase in advanced interventions and at least one institution reported a reduction in all-cause mortality [14]. The MAPPET 3 and PEITHO trials demonstrated reduction in all-cause mortality, but at the cost of increased stroke, extracranial, and intracranial bleeding [7, 23]. Potential mitigation of the bleeding risk was explored in MOPPET by using a lower dose of tissue plasminogen activator [8]. Whereas bleeding risk was lower, there was no significant difference in the individual outcomes of death and recurrent PE. All-cause mortality, however, can be an insensitive endpoint especially when examining a discrete and specific intervention such as thrombolysis/thrombectomy. The low rate of cause-specific mortality combined with high rate of comorbidity related mortality in this cohort creates pause when considering the benefit of PE-specific advanced interventions. Further assessment of risk factors leading to hemodynamic decompensation and mortality could better inform clinicians to optimize and individualize advanced therapies for PE.
A strategy geared towards advanced interventions in the IRPE group may still be reasonable if other clinically relevant outcomes could be positively influenced. Clinicians can then select from a range of therapies to individualize patient care. Some of these outcomes may include safety of intervention, residual dyspnea, RV dysfunction, clinical decompensation from intermediate risk PE, post-PE syndrome, and chronic thromboembolic pulmonary hypertension (CTEPH) [24]. Existing studies examining these outcomes have not supported widespread use of systemic thrombolysis and advanced interventions. Long term follow-up of the PEITHO study found that thrombolytic treatment did not affect long term residual dyspnea or RV dysfunction [9, 25]. Catheter directed thrombolytic strategies have to date examined only short term efficacy measures [26,27,28]. In the absence of significant PE specific mortality in the IRPE group, more studies exploring clinically meaningful outcomes of advanced interventions are needed.
This single-centered retrospective analysis has inherent limitations. The rate of autopsy examination was low and the determination of mortality attributable to PE was dependent largely on clinician determination. However, we believe this is a reasonable measure as the clinical picture of PE directly causing mortality is unambiguous. Of note, these data were obtained prior to the Coronavirus Disease 2019 (COVID-19) pandemic and may not necessarily apply to that population. However, given the overall high prevalence of PE, this study is still instructive when considering therapeutic options in non-COVID-19 patients. The rate of active malignancy was high in our cohort compared to the major prospective trials (36.8% vs. 6–15%), but similar to other major institutions reporting on their PERT experience [7, 8, 12, 23]. After the initial hospitalization, mortality was largely cancer related, suggesting PE in the setting of active malignancy is likely a poor prognostic factor. Further studies are needed to evaluate this hypothesis. Specific risk factors to predict decompensation in the intermediate risk group were unable to be identified in this study due to the low incidence. Likewise, the risks and benefits of catheter-based interventions could not be assessed due to their rare usage.
In summary, this study provides valuable insight into PE cause-specific mortality, which was found to be very low. The real world setting of our study can be more directly applicable to patients and can help inform clinicians and PERTs to have careful consideration of the types of outcomes expected when using advanced therapies in the intermediate risk group. A conservative treatment strategy largely using anticoagulation alone resulted in a very low cause-specific mortality in this study. Larger cohorts are needed to further assess risk factors associated with mortality and clinical decompensation in the intermediate risk group.
References
Wiener RS, Schwartz LM, Woloshin S (2011) Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med 171(9):831–836. doi:https://doi.org/10.1001/archinternmed.2011.178
Virani SS, Alonso A, Benjamin EJ et al (2020) Heart disease and stroke statistics—2020 update: a report From the American Heart Association. Circulation 141(9):E139–E596. https://doi.org/10.1161/CIR.0000000000000757
Smith SB, Geske JB, Kathuria P et al (2016) Analysis of national trends in admissions for pulmonary embolism. Chest 150(1):35–45. https://doi.org/10.1016/j.chest.2016.02.638
Duffett L, Castellucci LA, Forgie MA (2020) Pulmonary embolism: update on management and controversies. BMJ 370:m2177. doi:https://doi.org/10.1136/bmj.m2177
Kearon C, Akl EA, Ornelas J et al (2016) Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 149(2):315–352. doi:https://doi.org/10.1016/j.chest.2015.11.026
Jaff MR, McMurtry MS, Archer SL et al (2011) Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 123(16):1788–1830. https://doi.org/10.1161/CIR.0b013e318214914f
Meyer G, Vicaut E, Danays T et al (2014) Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 370(15):1402–1411. https://doi.org/10.1056/NEJMoa1302097
Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M (2013) Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” trial). Am J Cardiol 111(2):273–277. https://doi.org/10.1016/j.amjcard.2012.09.027
Konstantinides SV, Vicaut E, Danays T et al (2017) Impact of thrombolytic therapy on the long-term outcome of intermediate-risk pulmonary embolism. J Am Coll Cardiol 69(12):1536–1544. https://doi.org/10.1016/j.jacc.2016.12.039
Hao Q, Dong BR, Yue J, Wu T, Liu GJ (2018) Thrombolytic therapy for pulmonary embolism. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004437.pub5
Jimenez D, Martin-Saborido C, Muriel A et al (2018) Efficacy and safety outcomes of recanalisation procedures in patients with acute symptomatic pulmonary embolism: systematic review and network meta-analysis. Thorax 73(5):464–471. doi:https://doi.org/10.1136/thoraxjnl-2017-210040
Mahar JH, Haddadin I, Sadana D et al (2018) A pulmonary embolism response team (PERT) approach: initial experience from the Cleveland Clinic. J Thromb Thrombolysis 46(2):186–192. doi:https://doi.org/10.1007/s11239-018-1686-2
Kabrhel C, Jaff MR, Channick RN, Baker JN, Rosenfield K (2013) A multidisciplinary pulmonary embolism response team. Chest 144(5):1738–1739. https://doi.org/10.1378/chest.13-1562
Chaudhury P, Gadre S, Schneider E et al (2019) Impact of multidisciplinary pulmonary embolism response team availability on management and outcomes. Am J Cardiol 124(9):1465–1469. https://doi.org/10.1016/j.amjcard.2019.07.043
Jiménez D, de Miguel-Díez J, Guijarro R et al (2016) Trends in the management and outcomes of acute pulmonary embolism. J Am Coll Cardiol 67(2):162–170. https://doi.org/10.1016/j.jacc.2015.10.060
Donzé J, Gal G, Fine MJ et al (2008) Prospective validation of the Pulmonary Embolism Severity Index. Thromb Haemost 100(05):943–948. doi:https://doi.org/10.1160/TH08-05-0285
Rali PM, Criner GJ (2018) Submassive pulmonary embolism. Am J Respir Crit Care Med 198(5):588–598. https://doi.org/10.1164/rccm.201711-2302CI
Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis (2005) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 3(4):692–694. doi:https://doi.org/10.1111/j.1538-7836.2005.01204.x
Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W (2010) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 8(1):202–204. doi:https://doi.org/10.1111/j.1538-7836.2009.03678.x
Sy E, Sklar MC, Lequier L, Fan E, Kanji HD (2017) Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation: a systematic review and meta-analysis. J Crit Care 39:87–96. https://doi.org/10.1016/j.jcrc.2017.02.014
Sista AK, Goldhaber SZ, Vedantham S et al (2016) Research priorities in submassive pulmonary embolism: proceedings from a multidisciplinary research consensus panel. J Vasc Interv Radiol 27(6):787–794. https://doi.org/10.1016/j.jvir.2016.03.035
Aujesky D, Obrosky DS, Stone RA et al (2005) Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 172(8):1041–1046. doi:https://doi.org/10.1164/rccm.200506-862OC
Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W (2002) Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 347(15):1143–1150. https://doi.org/10.1056/NEJMoa021274
Sista AK, Horowitz JM, Goldhaber SZ (2016) Four key questions surrounding thrombolytic therapy for submassive pulmonary embolism. Vasc Med (United Kingdom) 21(1):47–52. doi:https://doi.org/10.1177/1358863X15614388
Goldhaber SZ (2017) PEITHO long-term outcomes study. J Am Coll Cardiol 69(12):1545–1548. https://doi.org/10.1016/j.jacc.2017.01.027
Piazza G, Hohlfelder B, Jaff MR et al (2015) A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism. JACC Cardiovasc Interv 8(10):1382–1392. https://doi.org/10.1016/j.jcin.2015.04.020
Kucher N, Boekstegers P, Müller OJ et al (2014) Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 129(4):479–486. doi:https://doi.org/10.1161/CIRCULATIONAHA.113.005544
Tapson VF, Sterling K, Jones N et al (2018) A Randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc Interv 11(14):1401–1410. https://doi.org/10.1016/j.jcin.2018.04.008
Funding
No funds, grants, or other support was received for conducting this study or to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This research study was conducted retrospectively from data obtained for clinical purposes. The study protocol was reviewed by the Institutional Review Board of Mayo Clinic and it was deemed exempt from the requirement of IRB approval (45 CFR 46.104d, category 4).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mudrakola, H.V., Caples, S.M., Hyde, R.J. et al. Inpatient Management of Pulmonary Embolism: Clinical Characteristics and Mortality in a High-Volume Tertiary Care Center. J Thromb Thrombolysis 54, 145–152 (2022). https://doi.org/10.1007/s11239-021-02619-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-021-02619-9