Abstract
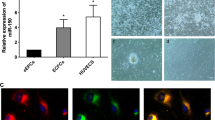
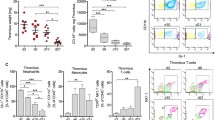
To assess the therapeutic efficacy of PDGF-D-overexpressing endothelial progenitor cells (EPCs) in deep vein thrombosis. Inferior vena cava thrombosis was induced in female Sprague Dawley (SD) rats. Animals were injected via the distal vena cava with EPCs overexpressing PDGF-D after transfection with a lentiviral vector containing the PDGF-D gene. The effect on thrombosis in animals who received EPCs was evaluated using MSB staining, immunohistochemistry, immunofluorescence, and venography; the steady-state mRNA and protein levels of PDGF-D and its receptor (PDGF-Rβ) were determined by RT-PCR and Western blotting, respectively; and the PDGF-D-induced mobilization of circulating EPCs was estimated by flow cytology. Compared with controls, injection of EPCs overexpressing PDGF-D was associated with increased thrombosis resolution; recanalization; PDGF-D and PDGF-Rβ expression; induction of monocyte homing; and mobilization of EPCs to the venous circulation. In a rat model, transplantation of PDGF-D-overexpressing EPCs facilitated the resolution of deep vein thrombosis.








Similar content being viewed by others
Data availability
The datasets generated/analyzed during the current study are available.
References
Heijboer H, Jongbloets LM, Büller HR, Lensing AW, ten Cate JW (1992) Clinical utility of real-time compression ultrasonography for diagnostic management of patients with recurrent venous thrombosis. Acta Radiol 33:297–300
Prandoni P, Cogo A, Bernardi E, Villalta S, Polistena P, Simioni P et al (1993) A simple ultrasound approach for detection of recurrent proximal-vein thrombosis. Circulation 88:1730–1735. https://doi.org/10.1161/01.cir.88.4.1730
Meissner MH, Manzo RA, Bergelin RO, Strandness DE (1994) Venous diameter and compliance after deep venous thrombosis. Thromb Haemost 72:372–376. https://pubmed.ncbi.nlm.nih.gov/7855786/
Arcelus JI, Caprini JA, Hoffman KN, Fink N, Size GP, Fareed J et al (1996) Laboratory assays and duplex scanning outcomes after symptomatic deep vein thrombosis: preliminary results. J Vasc Surg 23:616–621. https://doi.org/10.1016/s0741-5214(96)80041-3
Killewich LA, Macko RF, Cox K, Franklin DR, Benjamin ME, Lilly MP et al (1997) Regression of proximal deep venous thrombosis is associated with fibrinolytic enhancement. J Vasc Surg 26:861–868. https://doi.org/10.1016/s0741-5214(97)70101-0
Kahn SR, Ginsberg JS (2004) Relationship between deep venous thrombosis and the postthrombotic syndrome. Arch Intern Med 164:17–26. https://doi.org/10.1001/archinte.164.1.17
Henke PK, Varma MR, Moaveni DK, Dewyer NA, Moore AJ, Lynch EM, et al (2007) Fibrotic injury after experimental deep vein thrombosis is determined by the mechanism of thrombogenesis. Thromb Haemost. 98:1045–1055. https://pubmed.ncbi.nlm.nih.gov/18000610/
Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ (2008) Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133:454S-545S. https://doi.org/10.1378/chest.08-0658
Sevitt S (1973) The vascularisation of deep-vein thrombi and their fibrous residue: a post mortem angio-graphic study. J Pathol 111:1–11. https://doi.org/10.1002/path.1711110102
Modarai B, Burnand KG, Humphries J, Waltham M, Smith A (2005) The role of neovascularisation in the resolution of venous thrombus. Thromb Haemost. 93:801–809. https://doi.org/10.1160/THo4-09-0596
Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95:343–353. https://doi.org/10.1161/01.res.0000137877.89448.78
Meng Q, Wang W, Yu X, Li W, Kong L, Qian A et al (2015) Upregulation of MicroRNA-126 contributes to endothelial progenitor cell function in deep vein thrombosis via its target PIK3R2. J Cell Biochem 116:1613–1623. https://doi.org/10.1002/jcb.25115
Wang W, Li C, Li W, Kong L, Qian A, Hu N et al (2014) MiR-150 enhances the motility of EPCs in vitro and promotes EPCs homing and thrombus resolving in vivo. Thromb Res 133:590–598. https://doi.org/10.1016/j.thromres.2013.12.038
Ott I, Keller U, Knoedler M, Götze KS, Doss K, Fischer P et al (2005) Endothelial-like cells expanded from CD34+ blood cells improve left ventricular function after experimental myocardial infarction. Faseb J 19:992–994. https://doi.org/10.1096/fj.04-3219fje
Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD (2002) Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105:93–98. https://doi.org/10.1161/hc0102.101442
Li W-D, Li X-Q (2016) Endothelial progenitor cells accelerate the resolution of deep vein thrombosis. Vasc Pharmacol 83:10–16. https://doi.org/10.1016/j.vph.2015.07.007
Hur J, Yoon C-H, Kim H-S, Choi J-H, Kang H-J, Hwang K-K et al (2004) Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 24:288–293. https://doi.org/10.1161/01.ATV.0000114236.77009.06
Rehman J, Li J, Orschell CM, March KL (2003) Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107:1164–1169. https://doi.org/10.1161/01.cir.0000058702.69484.a0
Henke PK, Wakefield TW, Kadell AM, Linn MJ, Varma MR, Sarkar M et al (2001) Interleukin-8 administration enhances venous thrombosis resolution in a rat model. J Surg Res 99:84–91. https://doi.org/10.1006/jsre.2001.6122
Brindle NPJ, Saharinen P, Alitalo K (2006) Signaling and functions of angiopoietin-1 in vascular protection. Circ Res 98:1014–1023. https://doi.org/10.1161/01.RES.0000218275.54089.12
Miyata T, Iizasa H, Sai Y, Fujii J, Terasaki T, Nakashima E (2005) Platelet-derived growth factor-BB (PDGF-BB) induces differentiation of bone marrow endothelial progenitor cell-derived cell line TR-BME2 into mural cells, and changes the phenotype. J Cell Physiol 204:948–955. https://doi.org/10.1002/jcp.20362
Wakefield TW, Linn MJ, Henke PK, Kadell AM, Wilke CA, Wrobleski SK et al (1999) Neovascularization during venous thrombosis organization: a preliminary study. J Vasc Surg 30:885–892. https://doi.org/10.1016/s0741-5214(99)70013-3
Singh I, Burnand KG, Collins M, Luttun A, Collen D, Boelhouwer B et al (2003) Failure of thrombus to resolve in urokinase-type plasminogen activator gene-knockout mice: rescue by normal bone marrow-derived cells. Circulation 107:869–875
Modarai B, Burnand KG, Sawyer B, Smith A (2005) Endothelial progenitor cells are recruited into resolving venous thrombi. Circulation 111:2645–2653. https://doi.org/10.1161/CIRCULATIONAHA.104.492678
Cao Y (2013) Multifarious functions of PDGFs and PDGFRs in tumor growth and metastasis. Trends Mol Med 19:460–473. https://doi.org/10.1016/j.molmed.2013.05.002. (https://doi.org/10.1161/01.cir.0000050149.22928.39)
Demoulin J-B, Essaghir A (2014) PDGF receptor signaling networks in normal and cancer cells. Cytokine Growth Factor Rev 25:273–283. https://doi.org/10.1016/j.cytogfr.2014.03.003
Heldin C-H, Lennartsson J (2013) Structural and functional properties of platelet-derived growth factor and stem cell factor receptors. Cold Spring Harb Perspect Biol 5:a009100–a009100. https://doi.org/10.1101/cshperspect.a009100
Andrae J, Gallini R, Betsholtz C (2008) Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22:1276–1312. https://doi.org/10.1101/gad.1653708
Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH et al (2001) PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol 3:512–516. https://doi.org/10.1038/35074588
Ye Y, Li X, Zhang Y, Shen Z, Yang J (2016) Androgen modulates functions of endothelial progenitor cells through activated Egr1 signaling. Stem Cells Int 2016:7057894–7057916. https://doi.org/10.1155/2016/7057894
Humphries J, McGuinness CL, Smith A, Waltham M, Poston R, Burnand KG (1999) Monocyte chemotactic protein-1 (MCP-1) accelerates the organization and resolution of venous thrombi. J Vasc Surg 30:894–899. https://doi.org/10.1016/s0741-5214(99)70014-5
Clark RA (1993) Biology of dermal wound repair. Dermatol Clin 11:647–666
McGuinness CL, Humphries J, Waltham M, Burnand KG, Collins M, Smith A (2001) Recruitment of labelled monocytes by experimental venous thrombi. Thromb Haemost 85:1018–1024
Soo KS, Northeast AD, Happerfield LC, Burnand KG, Bobrow LG (1996) Tissue plasminogen activator production by monocytes in venous thrombolysis. J Pathol 178:190–194. https://doi.org/10.1002/(SICI)1096-9896(199602)178:2%3c190::AID-PATH454%3e3.0.CO;2-3
Knighton DR, Fiegel VD (1989) Macrophage-derived growth factors in wound healing: regulation of growth factor production by the oxygen microenvironment. Am Rev Respir Dis 140:1108–1111. https://doi.org/10.1164/ajrccm/140.4.1108
Nehls V, Herrmann R (1996) The configuration of fibrin clots determines capillary morphogenesis and endothelial cell migration. Microvasc Res 51:347–364. https://doi.org/10.1006/mvre.1996.0032
Lim BCB, Ariëns RAS, Carter AM, Weisel JW, Grant PJ (2003) Genetic regulation of fibrin structure and function: complex gene-environment interactions may modulate vascular risk. Lancet 361:1424–1431. https://doi.org/10.1016/S0140-6736(03)13135-2
Majno G, Joris I (1996) Cells, tissues, and disease. Principles of general pathology. Blackwell Science, Hoboken
Cox JS (1963) The maturation and canalization of thrombi. Surg Gynecol Obstet 116:593–599
Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M et al (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85:221–228. https://doi.org/10.1161/01.res.85.3.221
Ferrari N, Glod J, Lee J, Kobiler D, Fine HA (2003) Bone marrow-derived, endothelial progenitor-like cells as angiogenesis-selective gene-targeting vectors. Gene Ther. 10:647–656. https://doi.org/10.1038/sj.gt.3301883
Meng Q, Li X, Yu X, Lei F, Jiang K, Li C (2011) Transplantation of ex vivo expanded bone marrow-derived endothelial progenitor cells enhances chronic venous thrombus resolution and recanalization. Clin Appl Thromb Hemost 17:E196-201. https://doi.org/10.1177/1076029610397180
Iwaguro H, Yamaguchi J-I, Kalka C, Murasawa S, Masuda H, Hayashi S-I et al (2002) Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation 105:732–738. https://doi.org/10.1161/hc0602.103673
Kong L, Hu N, Du X, Wang W, Chen H, Li W et al (2016) Upregulation of miR-483–3p contributes to endothelial progenitor cells dysfunction in deep vein thrombosis patients via SRF. J Transl Med. https://doi.org/10.1186/s12967-016-0775-2
Acknowledgements
We would like to express our sincere appreciation to the reviewers for their helpful comments on this article.
Funding
This work was supported by the Grant from the National Natural Science Foundation of China (81400348) and the Kuanren Talents Program of the second affiliated hospital of Chongqing Medical University.
Author information
Authors and Affiliations
Contributions
BT designed the experiments and wrote the manuscript. HZ performed most experiments and analyzed data. HL was involved in analyzing the data. YC helped in animal experiments. JS analyzed the histology and histopathology and interpreted data. Jian Fu designed and supervised all experiments and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Ethical approval
The ethics committee of The Second Affiliated Hospital of Chongqing University (Chongqing, China) provided ethical approval for the experiments involved animals were implemented in accordance with the principles embodied in the National Institutes of Health Guide for the Care and Use of Laboratory. Efforts were made to avoid all unnecessary painful to the animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, H., Luo, H., Tang, B. et al. Endothelial progenitor cells overexpressing platelet derived growth factor-D facilitate deep vein thrombosis resolution. J Thromb Thrombolysis 53, 750–760 (2022). https://doi.org/10.1007/s11239-021-02567-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-021-02567-4