Abstract
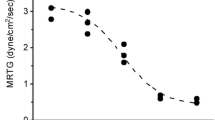
In recent years a variety of metals (cadmium, chromium, copper, iron) have been demonstrated to modulate coagulation in vitro and in vivo. One group of metals, the platinoids, have not been assessed, and such investigation is justified given the thromboembolic phenomena associated with platinum-based chemotherapy. Thus, the goal of the present investigation was to assess the effects of carboplatin, cisplatin (platinum compounds), NAMI-A, and ruthenium chloride (ruthenium compounds) on human plasmatic coagulation. Human plasma was exposed to clinically relevant, equimolar concentrations of the aforementioned platinum and ruthenium compounds, with changes in plasmatic coagulation assessed via thrombelastography. The first series of experiments demonstrated no significant modulation of coagulation by the platinum compounds, while NAMI-A demonstrated mild hypercoagulability and ruthenium chloride exerted marked hypercoagulability. A second series of experiments utilizing a variety of specialized modifications of thrombelastography focused on ruthenium chloride revealed that this compound enhances prothrombin activation. While the hypercoagulability associated with platinum compounds in vivo do not appear to have a basis in plasmatic biochemistry, it appears that ruthenium compounds can exert procoagulant properties by enhancing the common pathway of human plasmatic coagulation. Future investigation of Ru based chemotherapeutic agents in development to assess procoagulant activity as part of evaluating their potential clinical safety is warranted.







Similar content being viewed by others
References
Nielsen VG, Jacobsen WK (2016) Iron modulates the alpha chain of fibrinogen. Biometals 29:235–238
Nielsen VG, Pretorius E (2014) Iron and carbon monoxide enhance coagulation and attenuate fibrinolysis by different mechanisms. Blood Coagul Fibrinolysis 25:695–702
Nielsen VG, Pretorius E (2014) Iron-enhanced coagulation is attenuated by chelation: thrombelastographic and ultrastructural analysis. Blood Coagul Fibrinolysis 25:845–850
Jankun J, Landeta P, Pretorius E, Skrzypczak-Jankun E, Lipinski B (2014) Unusual clotting dynamics of plasma supplemented with iron(III). Int J Mol Med 33:367–372
Pretorius E, Bester J, Vermeulen N, Lipinski B (2013) Oxidation inhibits iron-induced blood coagulation. Curr Drug Targets 14:13–19
Nielsen VG, Ward TD, Ford PM (2018) Effects of cupric chloride on coagulation in human plasma: role of fibrinogen. J Thromb Thrombolysis 46:359–364
Venter C, Oberholzer HM, Bester J, van Rooy MJ, Bester MJ (2017) Ultrastructural, confocal and viscoelastic characteristics of whole blood and plasma after exposure to cadmium and chromium alone and in combination: an ex vivo study. Cell Physiol Biochem 43:1288–1300
Arbi S, Oberholzer HM, van Rooy MJ, Venter C, Bester MJ (2017) Effects of chronic exposure to mercury and cadmium alone and in combination on the coagulation system of Sprague-Dawley rats. Ultrastruct Pathol 41:275–283
van Rensburg MJ, van Rooy M, Bester MJ, Serem JC, Venter C, Oberholzer HM (2019) Oxidative and haemostatic effects of copper, manganese and mercury, alone and in combination at physiologically relevant levels: an ex vivo study. Hum Exp Toxicol 38:419–433
Elias TP (1969) An in vitro study of the effect of arsenic (As2O3) on blood clotting. Acta Haematol 41:239–248
Nielsen VG (2019) Lethal concentrations of mercury or lead do not affect coagulation kinetics in human plasma. J Thromb Thrombolysis 48:697–698
Kim ES, Baran AM, Mondo EL, Rodgers TD, Nielsen GC, Dougherty DW, Pandya KJ, Rich DQ, van Wijngaarden E (2017) Risk of thromboembolism in cisplatin versus carboplatin-treated patients with lung cancer. PLoS ONE 12:e0189410
Seng S, Liu Z, Chiu SK, Proverbs-Singh T, Sonpavde G, Choueiri TK, Tsao C, Yu M, Hahn NM, Oh WK, Galsky MD (2012) Risk of venous thromboembolism in patients with cancer treated with Cisplatin: a systematic review and meta-analysis. J Clin Oncol 30:4416
Gorodetsky R, Peylan-Ramu N, Reshef A, Gaberman E, Levdansky L, Marx G (2005) Interactions of carboplatin with fibrin(ogen), implications for local slow release chemotherapy. J Control Release 102:235–245
Ohta N, Yotsuyanagi T (1993) Alteration of fibrinogen secondary structure by cis-diamminedichloroplatinum(II) and calcium protection. Biol Pharm Bull 16:631–634
Alessio E, Messori L (2019) NAMI-A and KP1019/1339, two iconic ruthenium anticancer drug candidates face-to-face: a case story in medicinal inorganic chemistry. Molecules 24:1995
Chu AJ (2011) Polycations selectively blocking tissue factor-dependent FVII activation: collective in vitro anticoagulation studies. Inflamm Allergy Drug Targets 10:13–18
Khamrang T, Hung KC, Hsia CH, Hsieh CY, Velusamy M, Jayakumar T, Sheu JR (2017) Antiplatelet activity of a newly synthesized novel ruthenium (II): a potential role for Akt/JNK signaling. Int J Mol Sci 18:916
Hsia CH, Velusamy M, Sheu JR, Khamrang T, Jayakumar T, Lu WJ, Lin KH, Chang CC (2017) A novel ruthenium (II)-derived organometallic compound, TQ-6, potently inhibits platelet aggregation: ex vivo and in vivo studies. Sci Rep 7:9556
Nielsen VG (2020) Ruthenium, not carbon monoxide, inhibits the procoagulant activity of atheris, echis, and pseudonaja venoms. Int J Mol Sci 21:2970
Konings IR, Engels FK, Sleijfer S, Verweij J, Wiemer EA, Loos WJ (2009) Application of prolonged microdialysis sampling in carboplatin-treated cancer patients. Cancer Chemother Pharmacol 64:509-516A
Clerico A, Cappelli C, Ragni G, Caroli S, De Ioris MA, Sordi A, Petrucci F, Bocca B, Alimonti A (2006) Evaluation of carboplatin pharmacokinetics in pediatric oncology by means of inductively coupled plasma mass spectrometry. Ann Ist Super Sanità 42:461–468
Nielsen VG, Kirklin JK, Hoogendoorn H, Ellis TC, Holman WL (2007) Thrombelastographic method to quantify the contribution of factor XIII to coagulation kinetics. Blood Coagul Fibrinolysis 18:145–150
Nielsen VG, Gurley WQ Jr, Burch TM (2004) The impact of factor XIII on coagulation kinetics and clot strength determined by thrombelastography. Anesth Analg 99:120–123
Horowitz NA, Brenner B (2020) Thrombosis in hematological malignancies: mechanisms and implications. Thromb Res 191(Suppl 1):S58–S62
Funding
This investigation was supported by the Department of Anesthesiology, College of Medicine, at the University of Arizona.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethical approval
This was an in vitro investigation and did not involve any living subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nielsen, V.G. Platinoid effects on human plasmatic coagulation kinetics: a viscoelastic analysis. J Thromb Thrombolysis 51, 577–583 (2021). https://doi.org/10.1007/s11239-020-02373-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-020-02373-4