Abstract
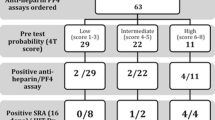
Heparin-induced thrombocytopenia (HIT) is a rare adverse drug reaction. The anti-PF4 antibody assay (ELISA) is utilized to assist in the clinical evaluation of HIT due to its high negative predictability and wide-spread availability. However, it also associated with false positive results. The 4T score can assist in predicting an individual’s risk for HIT and the need for further laboratory testing. This was a single-center prospective observational cohort study. Orders for HIT testing were sent via page to a clinical pharmacist to calculate a 4T score. If low risk, the pharmacist contacted the ordering prescriber to recommend discontinuation of laboratory testing. During the study, a clinical support tool was implemented to assist prescribers with ordering HIT tests. The study was divided into a pharmacist intervention group and a control group. A total of 303 pages were received. One hundred nine were missed due to unavailability of the pharmacist at time of page. A pharmacist reviewed 194 pages and intervened on 132. One hundred seven were scored as low risk, 70 as intermediate risk and 9 as high risk. Pharmacist intervention resulted in discontinuing 64 ELISA and 11 serotonin release assay tests. The clinical support tool resulted in a yearly decrease of HIT testing by 27%. Laboratory cost savings totaled $11,000 but did not include avoidance of laboratory technician or drug cost. Pharmacist involvement in the clinical assessment of HIT and the use of a support tool resulted in the reduction of HIT tests in low risk patients.
Similar content being viewed by others
References
Kasthuri RS, Glover SL, Jonas W et al (2012) PF4/heparin-antibody complex induces monocyte tissue factor expression and release of tissue factor positive microparticles by activation of FcγRI. Blood 119(22):5285–5293
Kelton JG, Sheridan D, Santos A et al (1988) Heparin-induced thrombocytopenia: laboratory studies. Blood 72(3):925–930
Greinacher A (2015) Heparin-induced thrombocytopenia. N Engl J Med 373(19):1883–1884. This is actually the citation for the commentary of the article: original article is 373(3):252–2651
Martel N, Lee J, Wells PS (2005) Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 106(8):2710–2715
Warkentin TE, Levine MN, Hirsh J et al (1995) Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 332(20):1330–1335
Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A (2006) Evaluation of pretest clinical score (4T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost 4(4):759–765
Warkentin TE, Greinacher A, Gruel Y, Aster RH, Chong BH (2011) Laboratory testing for heparin-induced thrombocytopenia: a conceptual framework and implications for diagnosis. J Thromb Haemost 9(12):2498–2500
Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y et al (2018) American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin induced thrombocytopenia. Blood Adv 2(22):3360–3392
Cuker A, Gimotty PA, Crowther MA, Warkentin TE (2012) Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood 120(20):4160–4167
Bayat M, Macedo FB, Ansari AS, Bracey AW, Akinyele S, Salazar M (2015) Evaluation of clinical and laboratory data for early diagnosis of heparin-induced thrombocytopenia. Am J Health Syst Pharm 72(19):1649–1655
Ruf KM, Bensadoun ES, Davis GA, Flynn JD, Lewis DA (2011) A clinical-laboratory algorithm incorporating optical density value to predict heparin-induced thrombocytopenia. Thromb Haemost 105(3):553–559
Wychowskil MK, Rusciol CI, Kouides PA, Sham RL (2017) The scope and value of an anticoagulation stewardship program. J Thromb Thrombolysis 43:380–386
Burnett AE, Bowles H, Borrego ME, Montoya TN, Garcia DA, Mahan C (2016) Heparin-induced thrombocytopenia: reducing misdiagnosis via collaboration between an inpatient anticoagulation pharmacy service and hospital reference laboratory. J Thromb Thrombolysis 42(4):471–478
Padron M, Miyares MA (2015) Development of an anticoagulation stewardship program at a large tertiary care academic institution. J Pharm Pract 28(1):93–98
Reardon DP, Atay JK, Ashley SW, Churchill WW, Berliner N, Connors JM (2015) Implementation of a hemostatic and antithrombotic Stewardship program. J Thromb Thrombolysis 40(3):379–382
Madden GR, German-Mesner I, Cox HL et al (2018) Reduced clostridium difficile tests and laboratory-identified events with a computerized clinical decision support tool and financial incentive. Infect Control Hosp Epidemiol 39:737–740
Hasan M, Malalur P, Agastya M et al (2016) A high-value cost conscious approach to minimize heparin induced thrombocytopenia antibody (HITAb) testing using the 4T score. J Thromb Thrombolysis 42(3):441–446
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Condon, A.J., Hood, A.J., Willenborg, K.L. et al. Pharmacist involvement in clinical assessment and laboratory testing for heparin-induced thrombocytopenia. J Thromb Thrombolysis 50, 195–200 (2020). https://doi.org/10.1007/s11239-019-02011-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-019-02011-8