Abstract
Deep venous thrombosis (DVT) is common in intensive care unit (ICU) patients. It is often silent and may be complicated by pulmonary embolism and death. Thromboprophylaxis with heparin does not always prevent venous thromboembolism (VTE). Aspirin (ASA) reduces the risk of VTE in surgical and high-risk medical patients but it is unknown if ASA may prevent DVT in mechanically ventilated ICU patients. We performed a retrospective chart review of critically ill patients who received mechanical ventilation for >72 h and underwent venous ultrasonography for suspected DVT between Jan 2012 and Dec 2013. We excluded patients who were on therapeutic doses of anticoagulation or had coagulopathy. We used multivariable logistic regression to evaluate association between aspirin use and DVT during hospitalization. There were 193 patients. The mean ± SD age was 58 ± 15.7 years. Half were male. DVT was found in 49 (25.4%). DVT was found in the first 15 days of hospitalization in 67.3% of the patients. The majority (82.8%) received thromboprophylaxis with unfractionated or low molecular weight heparin. Fifty-six (29%) were on ASA. On multivariable regression analysis, ASA use was associated with a significant reduction in the odds of finding DVT (OR 0.39, 95% CI 0.16–0.94; p = 0.036). DVT is common in mechanically ventilated ICU patients despite the use of thromboprophylaxis. Aspirin may prevent DVT in such patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deep venous thrombosis (DVT) is common in critically ill patients [1,2,3]. Such patients are especially vulnerable due to multiple risk factors: mechanical ventilation, immobilization, use of sedatives and paralytics, use of central venous catheters (CVC) [4], prior history of venous thromboembolism (VTE), end-stage renal disease, use of vasopressors, platelet transfusion [3], malignancy [2], morbid obesity [5], and congestive heart failure [6, 7]. Deep venous thrombosis may be complicated by pulmonary embolism (PE) and death. Deep venous thrombosis in critically ill patients is often clinically silent [8] and pharmacologic thromboprophylaxis with subcutaneous heparin is not always effective in preventing VTE in such patient [2, 3, 5]. Dalteparin was not found to be any better than unfractionated heparin in reducing DVT in critically ill patients in the PROphylaxis for ThromboEmbolism in Critical care Trial (PROTECT) [9]. Consequently, there is a need for a more effective method of pharmacologic thromboprophylaxis. Aspirin (ASA) has been shown to reduce the risk of VTE in surgical and high-risk medical patients [10]. However, it is unknown if ASA may prevent DVT in mechanically ventilated intensive care unit (ICU) patients, who remain at risk of VTE despite pharmacologic thromboprophylaxis. We hypothesized that rate of DVT would be lower in mechanically ventilated ICU patients who are on ASA compared to those who are not on it.
Materials and methods
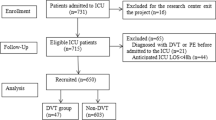
We performed a case control study of adult patients who were in an ICU between Jan 2012 and Dec 2013 and met the following inclusion criteria: received mechanical ventilation for >72 h and underwent venous ultrasonography for suspected DVT. We excluded patients who were on therapeutic anticoagulation. We retrospectively reviewed the electronic medical charts of the patients who met the inclusion criteria. The study (UFJ 2014-025) was approved by the Institutional Review Board of the University of Florida at Jacksonville.
We collected the following data: demographic characteristics including age, gender, body mass index (BMI); risk factors for DVT like CVC or peripherally inserted central venous catheters (PICC), active malignancy, prior history of VTE, sepsis; use of ASA; use of other anti-platelet agents; use of thromboprophylaxis; ventilator days; and hospital length of stay (LOS). ASA was administered either orally in patients who could tolerate or crushed and delivered via oro-gastric/nasogastric feeding tube in intubated patients.The diagnosis of DVT was based on the visualization of an intravascular thrombus, incompressibility of the vein by probe pressure, absence of spontaneous flow by Doppler, and absence of variation in flow with respiration. The diagnosis of DVT required direct visualization of the thrombus and one or more of the other signs in the deep veins of either upper or lower extremity. The CVCs used at our institution are made of oligon material and are heparin-coated. Catheter-related DVT was defined as DVT in an extremity vein in which CVC or PICC was in place at the time of diagnosis or within the preceding 72 h.
We reported categorical variables as numbers (percentages) for and continuous variables as means (standard deviation). Univariable regression analysis was performed for all the collected variables. Variables from univariable analysis were then used in a stepwise approach to perform multivariable logistic regression to evaluate association between ASA use and DVT. The model with the lowest Akaike’s Information Criterion was selected and used to estimate the odds ratio (OR) for the association. We defined statistical significance as p value <0.05. We also performed sensitivity analysis by excluding patients with catheter related DVT or history of VTE. We used Stata, version 12.1 (Stat Corp, College Station, Texas) to perform statistical analysis.
Results
There were a total of 193 patients. The mean ± SD age was 58 ± 15.7 years and 50% were male. Deep vein thrombosis was found in 49 (25.4%) patients. The characteristics of patients with and without DVT are compared in Table 1.
Deep venous thrombosis was catheter related in 17 (34.6%) patients. There were only six patients with active malignancy but DVT was found in half of them. There were only six patients with a prior history of VTE and DVT was found in only one of them.
All received mechanical thromboprophylaxis with an intermittent pneumatic compression device. The majority (n = 159, 82.8%) of patients received pharmacologic thromboprophylaxis in addition to mechanical thromboprophylaxis. All patients on pharmacological thromboprophylaxis received unfractionated heparin. Dose adjustment based on body weight was performed in morbidly obese patients (BMI > 40) as per the ICU protocol. There were 56 (29%) patients on ASA. Of the patients on pharmacological thromboprophylaxis, 48 (30.19%) were on aspirin and 39 (24.53%) experienced DVT (Table 2). The majority (n = 47, 83.9%) of those on ASA received 81 mg daily and the rest (n = 12, 17%) 325 mg daily. There were only 11 (5.7%) patients on dual antiplatelet therapy with ASA and Clopidogrel. Deep venous thrombosis was found in the first 15 days of hospitalization in the majority (67.3%) of the patients.
Deep venous thrombosis involved the upper extremity in 28 (57%) patients and the lower extremity in 21 (42.9%). The characteristics of patients with upper and lower extremity DVTs are compared in Table 3.
Patients with upper extremity DVT were older in age (60 vs. 56 years) and had a higher mean BMI (32 vs. 28) compared to patients with lower extremity DVT but statistical significance was not seen in any of these associations (Table 3).
On multivariable analysis, we found a statistically significant decrease in the odds of finding DVT in patients on ASA after adjusting for age, male gender, CVC (or PICC), sepsis and use of pharmacologic thromboprophylaxis (OR 0.39, 95%CI 0.16–0.95; p = 0.039) (Table 4).
This association remained even after excluding catheter related DVTs. Deep venous thrombosis was not found in any of the 11 patient who were on both ASA and Clopidogrel.
Discussion
Our study confirms that DVT is common in mechanically ventilated ICU patients despite the use of thromboprophylaxis, and suggests that ASA may prevent DVT in such patients. The 25% rate of DVT in our study is similar to the rate found in a study of mechanically ventilated medical ICU patients in whom pharmacologic thromboprophylaxis was universal [2]. Similarly, the majority of DVTs in that study was also found in the first 2 weeks of hospitalization. However, that study did not look into any possible association between use of ASA and DVT.
The use of ASA was associated with a significant reduction in the odds of DVT in mechanically ventilated ICU patients in our study. There is a possibility that this association may not be causal given the retrospective nature of our study. However, reduction in the rate of DVT with the use of ASA is supported by the medical literature. There is growing evidence of the role of platelets in venous thrombosis. The amount of platelets in venous thrombi is relatively low in comparison to that of red cells and leukocytes. However, it has been shown that in platelet-depleted mice venous thrombi are not formed [11]. Platelets play a role in the formation of venous thrombi by releasing polyphosphates and pro-inflammatory mediators, phosphatidylserine and/or tissue factor-exposing microparticles, as well as by stimulating the formation of the neutrophils extracellular traps [12]. The latter provide a scaffold and a stimulus for platelet adhesion and thrombus formation [13]. Increased spontaneous platelet aggregation and circulating platelets aggregates have been shown in patients with idiopathic recurrent DVT [14].
The PE prevention (PEP) trial [15], together with a previous meta-analysis by the Anti-platelet Trialists’ Collaboration (ATC) showed that, in orthopedic, general surgical and high-risk medical patients, ASA reduced the risk of DVT and PE by at least a third, largely irrespective of the use of any other thromboprophylaxis (including subcutaneous heparins) [16]. The small number of high-risk medical patients consisted of those who suffered an acute myocardial infarction (MI) or stroke. Aspirin is recommended as an option for thromboprophylaxis after major orthopedic surgery [17, 18]. In another meta-analysis that included only recent studies, the ATC showed that, in high-risk medical patients, ASA reduced the risk of PE by 25%. High-risk medical patients consisted of those at increased risk of occlusive vascular events i.e., those with an acute MI or ischemic stroke, unstable or stable angina, previous MI, stroke or cerebral ischemia, peripheral arterial disease, or atrial fibrillation [19]. More recently, a combined analysis of WARFASA and ASPIRE trials showed that ASA reduced the rate of recurrent VTE by one-third in patients with unprovoked VTE who had completed initial treatment with heparin followed by warfarin for a minimum of 6 weeks [20].
Nevertheless, only a placebo-controlled randomized clinical trial can definitively prove if ASA can reduce the risk of DVT and PE in critically ill patients. Such a trial should be feasible since ASA is widely available and inexpensive, and such a trial would be worthwhile for a number of reasons. Deep venous thrombosis in critically ill patients is often clinically silent [8] and may prove fatal. Pharmacologic thromboprophylaxis with subcutaneous heparin is not always effective in preventing VTE in critically ill patients [2, 3, 5]. The subcutaneous route may be unreliable in such patients [21] especially with the use of vasopressors [22]. The PROTECT clinical collaborators also reported that failure of pharmacologic thromboprophylaxis is more likely in obese ICU patients [5]. Critically ill patients who develop VTE have longer ICU and hospital LOS which contribute to hospital costs, morbidity and mortality [23].
Our study is not without limitations. It is retrospective and small. It does not exclude the possibility of prevalent DVTs at the time of admission to ICU since patients were not screened. However, it is unlikely this would have affected the results since only 3% of DVTs were prevalent DVTs in a prospective study of the prevalence, incidence, and risk factors for proximal lower extremity DVT among critically ill medical-surgical patients in whom pharmacologic thromboprophylaxis was universal [3]. Our study did not look into the rate of PE—an outcome that is more important than DVT. Since ICU patients who are found to have DVT may not be evaluated for PE even when suspected PE is the reason for performing venous ultrasonography, the rate of PE would have been low and our study sample would have been too small to find any association between use of ASA and PE. Moreover, a small proportion (17%) of patients in our study were not on any pharmacologic thromboprophylaxis. However, all patients received mechanical prophylaxis with an intermittent pneumatic compression device. More importantly, our study did not evaluate the possibility of bleeding complications associated with use of ASA in critically ill patients. Anti-platelet agents were found to be a risk factor for major bleeding in critically ill patients receiving pharmacologic thromboprophylaxis in PROTECT [24]. However, the majority of patients in our study was on low dose ASA.
Conclusions
Our study suggests that ASA may potentially reduce the rate of VTE beyond what can be achieved with pharmacologic thromboprophylaxis in mechanically ventilated ICU patients. The use of this widely available and inexpensive drug in critically ill patients to prevent an easily overlooked and potentially fatal disease as VTE should be further evaluated with randomized clinical trials.
References
Cade JF (1982) High risk of the critically ill for venous thromboembolism. Crit Care Med 10(7):448–450
Ibrahim EH, Iregui M, Prentice D, Sherman G, Kollef MH, Shannon W (2002) Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis. Crit Care Med 30(4):771–774
Cook D, Crowther M, Meade M, Rabbat C, Griffith L, Schiff D et al (2005) Deep venous thrombosis in medical-surgical critically ill patients: prevalence, incidence, and risk factors. Crit Care Med 33(7):1565–1571
Cook D, Attia J, Weaver B, McDonald E, Meade M, Crowther M (2000) Venous thromboembolic disease: an observational study in medical-surgical intensive care unit patients. J Crit Care 15(4):127–132
Lim W, Meade M, Lauzier F, Zarychanski R, Mehta S, Lamontagne F et al (2015) Failure of anticoagulant thromboprophylaxis: risk factors in medical-surgical critically ill patients*. Crit Care Med 43(2):401–410
Beemath A, Stein PD, Skaf E, Al Sibae MR, Alesh I (2006) Risk of venous thromboembolism in patients hospitalized with heart failure. Am J Cardiol 98(6):793–795
Al-Saffar F, Gupta E, Siddiqi F, Faisal M, Jones LM, Seeram V et al (2015) Is there any association between PEEP and upper extremity DVT? Crit Care Res Pract 2015:614598
Crowther MA, Cook DJ, Griffith LE, Devereaux PJ, Rabbat CC, Clarke FJ et al (2005) Deep venous thrombosis: clinically silent in the intensive care unit. J Crit Care 20(4):334–340
Cook D, Meade M, Guyatt G, Group PIftCCCT, Australian t, Group NZICSCT, et al (2011) Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med 364(14):1305–1314
Becattini C, Agnelli G (2014) Aspirin for prevention and treatment of venous thromboembolism. Blood Rev 28(3):103–108
von Brühl M-L, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M et al (2012) Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 209(4):819–835
Brinkmann V, Zychlinsky A (2007) Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol 5(8):577–582
Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF et al (2012) Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost 10(1):136–144
Wu KK, Barnes RW, Hoak JC (1976) Platelet hyperaggregability in idiopathic recurrent deep vein thrombosis. Circulation 53(4):687–691
Rodgers A, MacMahon S, Collins R, Prentice C (2000) Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: pulmonary embolism prevention (PEP) trial. Lancet 355(9212):1295–1302
Antiplatelet Trialists’ Collaboration(1994) Collaborative overview of randomised trials of antiplatelet therapy-III: reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients. BMJ 308(6923):235–246
Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S et al (2012) Prevention of VTE in orthopedic surgery patients: antithrombotic Therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl):e278S–e325S
American Academy of Orthopaedic Surgeons Clinical Guideline on Prevention of Pulmonary Embolism in Patients Undergoing Total Hip or Knee Arthroplasty (2007). Available from: http://almacen-gpc.dynalias.org/publico/Prevencion Embolismo tras artroplastia AAOOS.pdf
Antithrombotic Trialists’ Collaboration (2002) Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324(7329):71–86
Simes J, Becattini C, Agnelli G, Eikelboom JW, Kirby AC, Mister R et al (2014) Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation 130(13):1062–1071
Priglinger U, Delle Karth G, Geppert A, Joukhadar C, Graf S, Berger R et al (2003) Prophylactic anticoagulation with enoxaparin: Is the subcutaneous route appropriate in the critically ill? Crit Care Med 31(5):1405–1409
Dörffler-Melly J, de Jonge E, Pont A-C, Meijers J, Vroom MB, Büller HR et al (2002) Bioavailability of subcutaneous low-molecular-weight heparin to patients on vasopressors. Lancet 359(9309):849–850
Chan CM, Shorr AF (2010) Venous thromboembolic disease in the intensive care unit. Semin Respir Crit Care Med 31(1):39–46
Lauzier F, Arnold DM, Rabbat C, Heels-Ansdell D, Zarychanski R, Dodek P et al (2013) Risk factors and impact of major bleeding in critically ill patients receiving heparin thromboprophylaxis. Intensive Care Med 39(12):2135–2143
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gupta, E., Siddiqi, F.S., Kunjal, R. et al. Association between aspirin use and deep venous thrombosis in mechanically ventilated ICU patients. J Thromb Thrombolysis 44, 330–334 (2017). https://doi.org/10.1007/s11239-017-1525-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-017-1525-x