Abstract
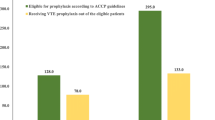
Transition from the hospital into the outpatient setting is a critical event for the appropriate provision of VTE prophylaxis. Data for this transition for the situation in Germany is scant. This was a retrospective, observational study in patients receiving in-hospital thromboprophylaxis and discharged with or without a recommendation to continue. Patient with previous thromboembolism were excluded. A total of 3,211 patients were identified by 518 physicians of which 2,853 had all data available for the present analysis; mean patient’s age was 57.4 ± 17.5 (SD) years, 48.2 % were male and bodyweight was 79.8 ± 16.1 kg. During hospitalization 95.5 % of surgical and 84.0 % of medical patients received any thromboprophylaxis, the mean hospital duration being 12.7 ± 20.3 days. Surgical patients had high, medium and low risk in 53.8, 37.1 and 9.1 %, respectively. Medical patients had high, medium and low risk in 78.8, 19.8 and 1.4 %. A hospital recommendation to continue thromboprophylaxis was given to 84.6 % (95 % CI 83.1–85.9 %) of surgical and 64.9 % (95 % CI 59.1–70.6 %) of medical patients and implemented in 96.6 and 94.3 %, respectively. On the other hand, in patients without a respective hospital recommendation (15.4 % of surgical and 35.1 % of medical patients), thromboprophylaxis was continued in 65.3 % of surgical and 73.1 % of medical patients because of high risk. Our data illustrate acceptable rates of prophylaxis in surgical and medical patients in Germany. As the results show, it is essential that not only hospital physicians are aware of the actual risk at discharge, but office based physicians assess thromboembolic risk.


Similar content being viewed by others
References
Encke A, Kopp I, Sauerland S. S3-Leitlinie Prophylaxe der venösen Thromboembolie (VTE). http://www.awmf.org/uploads/tx_szleitlinien/003-001l_S3_Thromboembolie-Prophylaxe_2010.pdf
Guyatt GH, Akl EA, Crowther M, Schunemann HJ, Gutterman DD, Zelman Lewis S, for the American College of Chest Physicians (2012) Introduction to the ninth edition: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141:48S–52S
Cohen AT, Tapson VF, Bergmann JF, Goldhaber SZ, Kakkar AK, Deslandes B, Huang W, Zayaruzny M, Emery L, Anderson FA, Jr., for the Endorse Investigators (2008) Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 371:387–394
Tapson VF, Decousus H, Pini M, Chong BH, Froehlich JB, Monreal M, Spyropoulos AC, Merli GJ, Zotz RB, Bergmann JF, Pavanello R, Turpie AG, Nakamura M, Piovella F, Kakkar AK, Spencer FA, Fitzgerald G, Anderson FA, Jr., for the Improve Investigators (2007) Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest 132:936–945
Kahn SR, Panju A, Geerts W, Pineo GF, Desjardins L, Turpie AG, Glezer S, Thabane L, Sebaldt RJ, for the Curve Study Investigators (2007) Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res 119:145–155
Arcelus JI, Felicissimo P, for the Deimos Investigators (2013) Venous thromboprophylaxis duration and adherence to international guidelines in patients undergoing major orthopaedic surgery: results of the international, longitudinal, observational DEIMOS registry. Thromb Res 131:e240–e246
Kaatz S, Spyropoulos AC (2011) Venous thromboembolism prophylaxis after hospital discharge: transition to preventive care. Hosp Pract (1995) 39:7–15
Casella G (1986) Refining binomial confidence intervals. Can J Stat 14:113–129
OECD. Health at a Glance 2011, OECD indicators: average length of stay in hospitals. (http://www.oecd-ilibrary.org/docserver/download/8111101ec033.pdf?expires=1380726176&id=id&accname=guest&checksum=A18B77AB779387BA84B53225E9EA8428)
Zotz RB, Kauschat-Bruning D, Bramlage P, for the Endorse Investigators (2009) Thromboembolic risk and prophylaxis in hospitalized surgical and internal medicine patients. German results of the international ENDORSE study. Dtsch Med Wochenschr 134:2163–2169
Kripalani S, Jackson AT, Schnipper JL, Coleman EA (2007) Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med 2:314–323
Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW (2007) Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA 297:831–841
Yusen RD, Hull RD, Schellong SM, Tapson VF, Monreal M, Samama MM, Chen M, Deslandes B, Turpie AG (2013) Impact of age on the efficacy and safety of extended-duration thromboprophylaxis in medical patients. Subgroup analysis from the EXCLAIM randomised trial. Thromb Haemost 110:1152–1163
Hull RD, Schellong SM, Tapson VF, Monreal M, Samama MM, Nicol P, Vicaut E, Turpie AG, Yusen RD, for the Exclaim Study (2010) Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med 153:8–18
Bergqvist D, Arcelus JI, Felicissimo P, for the Ethos Investigators (2012) Post-discharge compliance to venous thromboembolism prophylaxis in high-risk orthopaedic surgery: results from the ETHOS registry. Thromb Haemost 107:280–287
Rubenacker S, Kaiser J, Guschmann M (2013) Compliance of patients undergoing thromboprophylaxis with enoxaparin: the COMFORT study. Chirurg 84:235–242
Anderson FA, Jr., Goldhaber SZ, Tapson VF, Bergmann JF, Kakkar AK, Deslandes B, Huang W, Cohen AT, for the Endorse Investigators (2010) Improving practices in US hospitals to prevent venous thromboembolism: lessons from ENDORSE. Am J Med 123:1099-1106 e8
Colwell CW Jr, Pulido P, Hardwick ME, Morris BA (2005) Patient compliance with outpatient prophylaxis: an observational study. Orthopedics 28:143–147
Wilke T, Moock J, Muller S, Pfannkuche M, Kurth A (2010) Nonadherence in outpatient thrombosis prophylaxis with low molecular weight heparins after major orthopaedic surgery. Clin Orthop Relat Res 468:2437–2453
Acknowledgments
The statistical analysis provided by factum—Gesellschaft für Statistik, wissenschaftliche Information und Kommunikation, Offenbach, Germany is acknowledged. The study was funded and conducted by Sanofi-Aventis Deutschland GmbH, Berlin, Germany. SMS and PB have received research funding and honoraria for consultancy from Sanofi, the sponsor of the present study. JK is an employee of Sanofi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schellong, S.M., Kaiser, J. & Bramlage, P. Continuation of venous thromboembolism prophylaxis after hospital discharge into the outpatient setting: the ACCEPT study. J Thromb Thrombolysis 39, 173–178 (2015). https://doi.org/10.1007/s11239-014-1095-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-014-1095-0