Abstract
Eight species of Pandarus Leach, 1816 collected from hosts caught off South Africa are reported. These species include P. bicolor Leach, 1816, P. niger Kirtisinghe, 1950 and P. carcharhini Ho, 1963 belonging to the “bicolor” group and P. cranchii Leach, 1819, P. satyrus Dana, 1849, P. smithii Rathbun, 1886 and P. sinuatus Say, 1818 belonging to the “cranchii” group. Notes on previous and new distinguishing features are provided with illustrations, specifically the relative lengths of the dorsal plates and caudal rami as well as the structure of the distomedial spine on the second segment of leg 1 exopod. Additionally, illustrated re-descriptions are provided for P. satyrus and P. sinuatus. Furthermore, a new species Pandarus echinifer n. sp., also belonging to the “cranchii” group, collected from the snaggletooth shark Hemipristis elongata (Klunzinger) is described. This species is most similar to P. sinuatus but can be distinguished from it by the heavily spinulated distomedial spine on the last segment of the first leg exopod. Molecular analysis of the cytochrome oxidase I partial gene is used to calculate sequence divergences amongst different individuals and species. According to the results (as well as based on morphological characters) P. rhincodonicus Norman, Newbound & Knott, 2000 is a synonym of P. cranchii. New hosts and geographic localities from South Africa (and Ningaloo Park, Western Australia) are reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pandarus Leach, 1816 is one of the 23 genera of the family Pandaridae Milne Edwards, 1840 (Walter & Boxshall 2024) mostly infecting elasmobranchs, with Pandarus specimens commonly found on the body surface of the host (Cressey 1967; Kabata 1979). Currently there are 14 accepted species of Pandarus (Walter & Boxshall 2024), namely P. bicolor Leach, 1816; P. sinuatus Say, 1818; P. cranchii Leach, 1819; P. rouxii Risso, 1826; P. satyrus Dana, 1849; P. zygaenae Brady, 1883; P. brevicaudis Dana, 1852–1853; P. smithii Rathbun, 1886; P. ambiguus (Scott T., 1907); P. niger Kirtisinghe, 1950; P. carcharhini Ho, 1963; P. floridanus Cressey, 1967; P. katoi Cressey, 1967, and P. rhincodonicus Norman, Newbound & Knott, 2000. However, in the revision of the pandarids, Cressey (1967) made no mention of P. rouxii, P. brevicaudis or P. ambiguus. Carus (1885) referred to P. rouxii as a “species non determinanda” and thus should be a species inquirenda. Additionally, P. brevicaudis was accepted by Wilson (1907) as a valid species and described the female and male based on specimens described by Dana (1853) as P. brevicaudis and Nogagus validus, respectively. However, comparing Dana’s (1853) illustrations (see Figs. 2a and 3a on plate 95) of the females of P. satyrus and P. brevicaudis respectively, it is obvious that Fig. 3a represents an immature female. Wilson (1907) also mentioned P. brevicaudatus from Bassett-Smith (1899) as a synonym of P. brevicaudis which Bassett-Smith (1899) referred to as “imperfectly described”. Thus, P. brevicaudis should also be regarded as a species inquirenda. Regarding P. ambiguus described as Nogagus ambiguus in Scott & Scott (1913), the description and illustrations (see Figs. 1–8 of plate XX) is that of an immature pandarid (cf. Fig. 5A in Izawa (2010)), most likely a male due to the claw on the maxilliped (see Fig. 4). Comparing this description and illustration with those of the copepodid stages of male and female P. cranchii (Izawa 2010) discrepancies are observed especially regarding the structure of leg 4 of N. ambiguus (i.e. P. ambiguus) (see Fig. 8 of plate XX in Scott & Scott (1913)) with a 1-segmented exopod and 2-segmented endopod while leg 4 of the copepodid stages and adult female of P. cranchii have 1-segmented rami and the adult male has 2-segmented rami, similar to the adults of other Pandarus species (Cressey 1967). Therefore, P. ambiguus should also be regarded as a species inquirenda and consequently there are currently only 11 valid species.
According to Cressey (1967), the adult females can be divided into two groups based on their dorsal morphology, i.e., the “bicolor” group with the dorsal plates of thoracic somite two that extends only up to the posterior edge of the plate of somite three (including P. bicolor, P. niger, P. carcharini) and the “cranchii” group with the dorsal plates of the thoracic somite two extending well beyond the posterior edge of that of somite three (including P. sinuatus, P. cranchii, P. satyrus, P. zygaenae, P. smithii, P. floridanus, P. katoi and P. rhincodonicus). The three species in the “bicolor” group can be distinguished from each other mainly by the lengths of the caudal rami with those of P. bicolor barely visible in dorsal view, those of P. carcharhini just extending beyond the abdominal plate and those of P. niger extending well beyond the abdominal plate (Kirtisinghe 1950; Ho 1963; Cressey 1967). In the “cranchii” group, P. satyrus, P. cranchii and P. rhincodonicus are morphologically very similar (Cressey 1967; Norman et al. 2000) as are P. floridanus and P. sinuatus; and P. katoi and P. zygaenae (Cressey 1967) while P. smithii can be distinguished from all the other species in the “cranchii” group by the presence of basal medial expansions on the caudal rami (Izawa 2010). However, Pandarus species undergo considerable morphological changes during their ontogeny (Kabata 1979) as well as varying signs of pigmentation (Ho 1963; Cressey 1967; Kabata 1979) and therefore using their dorsal features for species identification may result in misidentifications. Additionally, the setation of the limbs also exhibits variability (Hewitt 1967) with some species having the same spine and setal formulas (Cressey 1967).
Current reports of Pandarus species from South African waters include P. bicolor from Carcharias sp., dogfish, Galeorhinus galeus (Linnaeus), Odontaspis sp., Squalus acanthias (Linnaeus) (Dippenaar 2004) and Carcharodon carcharias (Linnaeus), Mustelus palumbes Smith, Notorynchus cepedianus (Péron), and Triakis megalopterus (Smith) (Dippenaar 2024); P. carcharini from Carcharhinus leucas (Valenciennes) (Dippenaar 2004); P. niger from Carcharhinus obscurus (LeSueur) (Dippenaar 2024); P. cranchii from Carcharhinus longimanus (Poey), Carcharodon carcharias, Poroderma africanum (Gmelin), Sphyrna zygaena (Linnaeus), Stegostoma tigrinum (Foster), (Dippenaar 2004), Isurus oxyrinchus (Rafinesque) and Sphyrna lewini (Griffith & Smith) (Dippenaar 2024); P. floridanus from Carcharias taurus Rafinesque (Dippenaar 2004); and P. smithii from Carcharhinus sp., C. obscurus, Carcharias sp., C. taurus, Carcharodon carcharias, I. oxyrinchus, Odontaspis sp., Prionace glauca (Linnaeus), Rhincodon typus Smith, Rhizoprionodon acutus (Rüppell), (Dippenaar 2004), Carcharhinus brachyurus (Günther), C. limbatus, Galeocerdo cuvier (Péron & LeSueur), S. lewini (Dippenaar 2024).
In the present study, species of Pandarus were collected from elasmobranchs caught from both the Atlantic and Indian oceans off South Africa. Thus, the paper reports on eight Pandarus species collected with additional notes on their characteristic features and redescribes and illustrates the adult females of P. satyrus and P. sinuatus, including new hosts and/or geographical records of some collected species. Additionally, a new species, P. echinifer n. sp., is described. Sequences divergences of 13 individuals (10 downloaded from Genbank and three newly generated) using the cytochrome oxidase I partial gene were used to estimate inter- and intraspecific divergences within and amongst Pandarus species.
Materials and Methods
Sampling and morphological observation
Copepod specimens were collected from elasmobranch species caught mostly in the nets of the KwaZulu-Natal Sharks Board (KZNSB) and some species caught as by-catch during hake assessment demersal cruises off the west coast of South Africa on board the Department of Agriculture, Forestry and Fisheries (DAFF) research vessel R/V (Africana) during 2008 as well as from fish caught by commercial fishermen off Gansbaai (West coast). Additionally, specimens were collected from white sharks during the Ocearch Project in South Africa as well as from a snaggletooth shark from uShaka Marine World, Durban. Furthermore, specimens were collected from a stranded whale shark off the west coast while collected specimens from whale sharks swimming in Ningaloo Marine Park (Western Australia) were also obtained. The fish hosts were mostly identified by scientists at KZNSB and researchers on board the vessels. Collected specimens were fixed and preserved in 70% ethanol. Selected specimens were cleared and stained in lactic acid with a small amount of dissolved lignin pink. These specimens were dissected and studied under both stereo- and light microscopes using the wooden slide technique (Humes & Gooding 1964). Selected specimens were prepared for scanning electron microscopy (SEM) by dehydrating them through a series of ethanol (70, 80, 90, 100, 100% for about 30 min each) followed by immersion in hexamethyldisilazane for about an hour. Excess hexamethyldisilizane, not evaporated, were removed and the specimens were allowed to dry completely before being sputter-coated with gold-palladium and carbon. Host names were verified using Froese & Pauly (2024). Morphological nomenclature mostly follows Cressey (1967) and Kabata (1979). Voucher specimens and the type-material were deposited in the Iziko South African Museum, Cape Town, South Africa.
DNA extraction and sequencing
Genomic DNA was extracted from specimens of Pandarus collected from the stranded whale shark in South Africa as well as from specimens received from Ningaloo Marine Park (Western Australia) using the QIAGEN® QIAamp® DNA micro kit (Whitehead Scientific (Pty) Ltd) according to the manufacturer’s instructions. Polymerase Chain Reaction (PCR) was used to amplify a fragment of the mitochondrial (mtDNA) COI (Cytochrome Oxidase I) gene using primers LCO 1490 (forward) and HCO 2198 (reverse) (Folmer et al. 1994). The 20 µl PCR reaction mixture consisted of 10 µl Master mix (Ampliqon Taq DNA Polymerase Master Mix RED) containing HotStarTaq Plus DNA polymerase, 1 µl of each primer, 5–8 µl (depending on the DNA concentration) of DNA and ddH2O to top up the volume. The cycling conditions consisted of an initial denaturation at 95 °C (4 min); followed by 30 cycles of 94 °C (1 min) denaturation, 43–45 °C (2 min) annealing and 72 °C (3 min) extension, with a final extension of 72 °C (10 min) in the MiniOpticon real-time PCR system. Purification of PCR products and sequencing was done by Inqaba Biotechnical Industries (Pty) Ltd. The resulting chromatograms of the sequences were checked for nucleotide ambiguities, and the forward and reverse sequences assembled and edited using CLC main workbench 7.9.1 (QIAGEN). Generated sequences were aligned with sequences downloaded from Genbank (Accession numbers: HG942363, FJ447387–FJ447391, KJ551843 and OL457303–OL457305) using Clustal X 2.0.12 (Thompson et al. 1997). The aligned dataset was imported into MacClade 4.0 (Maddison & Maddison 2001) and translated into amino acids to verify the alignment. Uncorrected pairwise sequence divergences were estimated using MEGA 7 (Kumar et al. 2016). The generated sequences were submitted to Genbank (Accession numbers: PP434798–PP434800).
Results
Systematics
Family Pandaridae Milne Edwards, 1840
Genus Pandarus Leach, 1816
Pandarus bicolor Leach, 1816
Hosts: Mustelus palumbes Smith; M. mustelus (Linnaeus); Galeorhinus galeus (Linnaeus); Triakis megalopterus (Smith) (Carcharhiniformes: Triakidae); Notorynchus cepedianus (Péron) (Hexanchiformes: Hexanchidae), and Carcharodon carcharias (Linnaeus) (Lamniformes: Lamnidae) from west coast (SA).
Locality: Atlantic Ocean, South Africa.
Material examined: Several ♀♀ from all different host species.
Voucher material: Two adult ♀♀ (SAMC-A096821) collected from M. mustelus; four adult ♀♀ (SAMC-A096822) collected from G. galeus; two adult ♀♀ (SAMC-A096823) collected from N. cepedianus; two adult ♀♀ (SAMC-A096824) collected from C. carcharias.
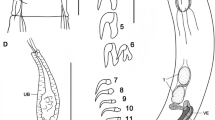
Pandarus bicolor females can be easily identified from all other species by a combination of the relative lengths of the dorsal thoracic plates and the structure of the caudal rami. The dorsal plates (Fig. 1a) of the second thoracic somite extend only to the posterior margin of those of the third thoracic somite (see Fig. 2 in Öktener et al. (2020) and Fig 769 in Kabata (1979)) while the caudal rami (Fig. 1b) are short and broad, barely visible in dorsal view (Fig. 1a) (see Fig. 3 in Öktener et al. (2020) and Fig 771 in Kabata (1979)).
Pandarus niger Kirtisinghe, 1950
Hosts: Carcharhinus obscurus (Lesueur) (Carcharhiniformes: Carcharhinidae) from east coast (SA).
Locality: Indian Ocean, South Africa.
Material examined: 1♀ from single host specimen.
Pandarus niger females are easily identified from all other species by the same combination of features as P. bicolor, namely the relative lengths of the dorsal thoracic plates and the appearance of the caudal rami. The dorsal plates of the second thoracic somite extend only to the posterior margin of those of the third thoracic somite (Fig. 1c) while the caudal rami are long and reach well beyond the posterior margin of the dorsal abdominal plate (Fig. 1c) (caudal rami about 11/3 times as long as the dorsal abdominal plate (Rangnekar & Rangnekar 1972) (see Plate 1 in Rangnekar & Rangnekar (1972)).
Pandarus carcharhini Ho, 1963
Hosts: Carcharhinus leucas (Valenciennes) (Carcharhiniformes: Carcharhinidae) from east coast (SA).
Locality: Indian Ocean, South Africa.
Material examined: 1♀ from single host specimen.
Pandarus carcharhini females are distinguished from all other species by the same combination of features as P. bicolor and P. niger, namely that the dorsal plates of the second thoracic somite extend only to the posterior margin of those of the third thoracic somite (Fig. 1d) while the caudal rami are longer than those in P. bicolor reaching beyond the posterior margin of the dorsal abdominal plate (Ho 1963) (although slightly shorter than abdominal plate in current specimen), but shorter than those of P. niger (see Fig. 13 in Ho (1963)).
Pandarus cranchii Leach, 1819
Hosts: Sphyrna lewini (Griffith & Smith) (Carcharhiniformes: Sphyrnidae); Carcharodon carcharias (Linnaeus); Isurus oxyrinchus Rafinesque (Lamniformes: Lamnidae) from east coast (SA), and Rhincodon typus Smith (Orectolobiformes: Rhincodontidae) from Ningaloo Marine Park (Western Australia).
Locality: Indian Ocean, South Africa and Ningaloo Marine Park (Western Australia).
Material examined: Several ♀♀ from all host specimens.
Voucher material: Four adult ♀♀ (SAMC-A096825) collected from S. lewini; one adult ♀ (SAMC-A096826) collected from C. carcharias; two adult ♀♀ (SAMC-A096827) collected from I. oxyrinchus; one adult ♀ (SAMC-A096828) collected from R. typus from Western Australia.
Pandarus cranchii females have dorsal plates of the second thoracic somite that extend well beyond the posterior margin of those of the third thoracic somite (Figs. 2a, b), sometimes even beyond the posterior margin of the plate of the fourth thoracic somite (Fig. 2a). The caudal rami extend mostly beyond the posterior margin of the dorsal abdominal plate (Fig. 2a) or at least up to the posterior margin (see Fig. 2b and Fig. 1A in Izawa (2010)). Additionally, the second segment of leg 1 exopod is armed with a distomedial spinulated process (Figs. 2c, d) (see Fig. 2A in Izawa (2010)).
Pandarus cranchii Leach, 1819. a, adult female from Sphyrna lewini (Griffith & Smith); b, adult female from Rhincodon typus Smith; c, scanning electron micrograph of distomedial spinulated process of leg 1 exopod distal segment; d, light microscope photo of distomedial spinulated process of leg 1 exopod distal segment.
Pandarus satyrus Dana, 1849
Hosts: Carcharodon carcharias (Linnaeus); Isurus oxyrinchus Rafinesque (Lamniformes: Lamnidae); Carcharias taurus Rafinesque (Lamniformes: Carchariidae) from east coast (SA), and Rhincodon typus Smith (Orectolobiformes: Rhincodontidae) from west coast (SA) and Ningaloo Marine Park (Western Austalia).
Locality: Indian and Atlantic Oceans, South Africa and Ningaloo Marine Park (Western Australia).
Material examined: Several ♀♀ from all host specimens.
Voucher material: Five adult ♀♀ (SAMC-A096829) collected from I. oxyrinchus; five adult ♀♀ (SAMC-A096830) collected from R. typus.
Re-description (Figs. 3, 4)
Adult female [based on 10 specimens]. Cephalothorax dorsally with several small denticles medially on posterior margin (Fig. 3a). Dorsal thoracic plates of second somite about twice as long as that of third somite reaching more than half length of dorsal plate of fourth somite (sometimes almost to posterior margin of dorsal plate of fourth somite (Fig. 3b)). Genital complex with paired posterior protrusions each armed with a small spine (Figs. 3c, d). Dorsal abdominal plate fan-shaped with small spinule on lateral margin (Fig. 3c). Caudal rami (Fig. 3e) lateral to dorsal abdominal plate, extending beyond middle of dorsal abdominal plate (Fig. 3a) (sometimes beyond posterior margin of dorsal abdominal plate (Fig. 3b)), sharply pointed distally with 2 processes and pinnate seta dorsally and 1 spine and small pinnate seta ventrally.
Pandarus satyrus Dana, 1849. a, adult female; b. posterior part of another specimen indicating lengths of dorsal plates and caudal rami; c, posterior part of genital complex, abdominal plate and caudal rami; d, posterior protrusion of genital complex with spine; e, distal part of caudal ramus; f, antennule, with enlarged setae (50 µm); g, antenna; h, mandible; i, maxillule; j, maxilla; k, leg 5. Scale-bars: a, b, 0.5 mm; c, 0.2 mm; d, 0.1 mm; e, f, g, j 100 µm; h, 10 µm; i, k, 50 µm.
Antennule (Fig. 3f), antenna (Figs. 3g, 4a), oral cone (Fig. 4b), mandible (Figs. 3h, 4b), maxillule (Figs. 3i, 4c), maxilla (Figs. 3j, 4d) and maxilliped (Fig. 4e) mostly similar to those of P. cranchii with small differences in the number of setae on the antennule and a possible additional small seta on the palp of the maxillule (not observed in all examined specimens).
Legs 1–3 biramous, 2-segmented, leg 4 1-segmented. Armature formula as follows with spines (Roman numerals) and setae (Arabic numerals):
Endopod | Exopod | |||
|---|---|---|---|---|
1 | 2 | 1 | 2 | |
Leg 1 | 0-0 | 3 | I-0 | III, I, 3 |
Leg 2 | 0-0 | 4 | I-0 | III, I, 6 |
Leg 3 | 0-0 | 2 | I-0 | III, I, 2 |
Leg 4 | 0(1) | - | IV, I, 1 |
Distomedial spine on leg 1 exopod bifid, shoe-shaped (Fig. 4f). Leg 5 (Fig. 3k) with inner conical process armed with small spine and outer small pinnate seta.
Remarks
Females of P. satyrus and P. cranchii are morphologically very similar and can apparently be distinguished by the lengths of the caudal rami relative to the length of the dorsal abdominal plate (Cressey 1967; Lewis 1966), while the leg armature is mostly the same for the two species (Cressey 1967). However, even though the plates of the second thoracic somite of P. cranchii mostly extends to or beyond the posterior margin of the plate of the fourth thoracic somite some individuals of P. satyrus have equally long plates of the second thoracic somite (current observations, see Fig. 3b). Furthermore, the caudal rami of P. satyrus mostly do not extend beyond the posterior margin of the dorsal abdominal plate, as often seen in P. cranchii (with exceptions, see Fig. 12D in Shiino (1954)), but some individuals also have equally long caudal rami as seen in P. cranchii (current observations, see Fig. 3b). Due to the variation in the armature of the legs present in members of Pandaridae (Hewitt 1967) reports of different numbers of armature elements are not unusual for P. satyrus females (e.g. Shiino 1954; Ho 1963; Lewis 1966; Cressey 1967; Hewitt 1967). However, a clear difference between P. satyrus and P. cranchii females is the structure of the distomedial spine on the last exopodal segment of leg 1 with that of P. cranchii being a spinulated process (see Figs. 2c, d and Fig. 2A in Izawa (2010), Fig. 13B in Shiino (1954), Fig. 12c in Lewis (1966) and Fig. 145 in Hewitt (1967)) while that of P. satyrus is a smooth, bifid process (see Fig. 4f and Fig. 9b in Lewis (1966)).
Pandarus smithii Rathbun, 1886
Hosts: Carcharhinus brachyurus (Günther) from west coast (SA); C. limbatus (Valeniennes); C. brevipinna (Valenciennes); C. obscurus (LeSueur) (Carcharhiniformes: Carcharhinidae); Galeocerdo cuvier (Péron & LeSueur) (Carcharhiniformes: Galeocerdonidae); Sphyrna lewini (Griffith & Smith) (Carcharhiniformes: Sphyrnidae) from east coast (SA); Carcharodon carcharias (Linnaeus) from east and west coasts (SA); Isurus oxyrinchus Rafinesque (Lamniformes: Lamnidae) from east coast (SA), and Rhincodon typus Smith (Orectolobiformes: Rhincodontidae) from west coast (SA).
Locality: Indian and Atlantic Oceans, South Africa.
Material examined: Several ♀♀ from all host specimens.
Voucher material: Six adult ♀♀ (SAMC-A096831) collected from C. carcharias; four adult ♀♀ (SAMC-A096832) collected from I. oxyrinchus.
Pandarus smithii females have dorsal plates of the second thoracic somite that extend beyond the posterior margin of those of the third thoracic somite, almost reaching middle of plate on fourth thoracic somite (Fig. 5a, see Fig. 34 in Cressey (1967) and Fig. 7A in Izawa (2010)). The caudal rami are mostly shorter than the dorsal abdominal plate or extend almost to the posterior margin of the dorsal abdominal plate (Fig. 5a, see Fig. 34 in Cressey (1967) and Fig. 7A in Izawa (2010)) bearing basal medial expansions (often shorter than posterior margin of abdomen (see Figs. 5b, 6a). The second segment of leg 1 exopod is armed with a distomedial smooth process with a slightly extended tip (Fig. 6b, c, d) (sometimes causing a slightly bifid appearance (Figs. 6c, d) (see Fig. 8A in Izawa (2010)).
Pandarus smithii Rathbun, 1886, scanning electron micrographs. a, abdomen and caudal rami, ventral view; b, smooth distomedial process with a slightly extended tip of leg 1 exopod distal segment; c, smooth distomedial process with a slightly extended tip of leg 1 exopod (different view); d, light microscope photo of distomedial smooth process of leg 1 exopod.
Pandarus sinuatus Say, 1818
Hosts: Carcharias taurus Rafinesque (Lamniformes: Carchariidae); Carcharhinus leucas (Valenciennes); C. plumbeus (Nardo) (Carcharhiniformes: Carcharhinidae); Sphyrna mokarran (Rüppell) (Carcharhiniformes: Sphyrnidae).
Locality: Indian Ocean, South Africa.
Material examined: Several ♀♀ from all host specimens.
Voucher material: 10 adult ♀♀ (SAMC-A096833) collected from C. taurus.
Re-description (Figs. 7, 8, 9)
Adult female [based on 10 specimens]. Body typical for Pandarus morphology. Cephalothorax dorsally with serrated posteromedial margin with about 4 sharp denticles on either side of middle (Fig. 7a). Dorsal thoracic plates of second somite slightly longer than that of third somite (sometimes appearing almost equal in length), reaching only about a third length of dorsal plate of fourth somite (Fig. 7a) (some specimens reaching about half length of dorsal plate of fourth somite (Fig. 7b)). Genital complex with paired posterior protrusions (Fig. 7a). Dorsal abdominal plate mostly circular with thin anterior extension joining with genital complex (Fig. 7a). Caudal rami (Figs. 7a, 8a) lateral to dorsal abdominal plate, extending slightly beyond middle of dorsal abdominal plate (Fig. 7a), slender, sharply pointed distally with 2 processes dorsally and 1 spine and small pinnate seta ventrally (Fig. 7c), slight basal medial expansions (Figs. 7a, 8a).
Pandarus sinuatus Say, 1818. a, adult female; b, dorsal thoracic plates of another specimen; c, caudal ramus; d, antennule; e, antenna; f, terminal claw of antenna; g, maxillule; h, maxilla; i, distal part of maxilla with calamus, canna and clavus. Scale-bars: a, b, 0.5 mm; c, h, 100 µm; d, e, f, g, i, 50 µm.
Pandarus sinuatus Say, 1818. a, scanning electron micrograph of abdomen and caudal rami, ventral view; b, scanning electron micrograph of maxillule; c, scanning electron micrograph of maxilliped; d, scanning electron micrograph of bulbous distomedial process of leg 1 exopod distal segment; e, scanning electron micrograph of distomedial bulbous process of leg 1 exopod distal segment (different view); f, light microscope photo of leg 1 exopod with distomedial bulbous process of distal segment and distal segment of endopod.
Antennule (Fig. 7d) 2-segmented with 22 and 11 setae on first and second segments, respectively. Antenna (Fig. 7e) similar to other Pandarus females with slightly reduced terminal claw (Fig. 7f). Oral cone and mandible similar to congeneric members. Maxillule (Figs. 7g, 8b) endite with stout distal process, palp with 2 (sometimes 3) small setae. Maxilla (Fig. 7h) brachiform, brachium with calamus, cana and clavus (Fig. 7i). Maxilliped (Fig. 8c) similar to congeneric members.
Legs 1–3 (Figs. 9a–d), biramous, 2-segmented, leg 4 (Fig. 9e) 1-segmented. Armature formula as follows with spines (Roman numerals) and setae (Arabic numerals):
Endopod | Exopod | |||
|---|---|---|---|---|
1 | 2 | 1 | 2 | |
Leg 1 | 0-0 | 3 | I-0 | III, I, 3 |
Leg 2 | 0-0 | 4 | I-0 | III, I, 6 |
Leg 3 | 0-0 | 2 | I-0 | III, I, 2 |
Leg 4 | 0 | - | IV, I, 1 |
Distomedial spine on leg 1 exopod smooth, bulbous ((Figs. 8d–f, 9a). Leg 5 (Fig. 9f) small, pointed tubercle and two small setae with adjacent pinnate seta.
Remarks
Females of P. sinuatus are part of the “cranchii group” with the dorsal plates of thoracic somite two extending “well beyond” the posterior margin of the plate of somite three (Cressey 1967) even though the plates of somite 2 only extend slightly beyond the plate of somite three. Furthermore, P. sinuatus is related to P. floridanus but are distinguishable by the lengths of the plates of thoracic somite 2, with those of P. floridanus extending beyond the middle of the plate of somite 4 whereas those of P. sinuatus are not extending to the middle of the plate of somite 4, but only to the anterior third (Cressey 1967). Additionally, the dorsal abdominal plate is about as long as the exposed caudal rami (Cressey 1967). However, current observations highlight the fact that the plates of thoracic somites two and three are often almost the same length (see Fig. 7a) while these plates may sometimes extend further than just the anterior third of the plate of somite four (see Fig. 7b). Additionally, the length of the caudal rami of the studied specimens were mostly shorter than the length of the dorsal abdominal plate (see Fig. 7a) rather than the same length (according to Wilson (1907)). The presence of slight basal medial expansions on the caudal rami of P. sinuatus individuals (see Figs. 7a, 8a) as well as the variation in the sizes and shapes of the dorsal thoracic plates resulted in difficulty distinguishing between females of P. sinuatus and P. smithii. However, the structure of the distomedial spine on the last exopodal segment of leg 1 can be used to distinguish the females of the two species, with that of P. sinuatus appearing much more bulbous (Figs. 8d–f) and not slightly bifid (Figs. 6b–d) like that of P. smithii.
Pandarus echinifer n. sp.
Type-host: Hemipristis elongata (Klunzinger) (Carcharhiniformes: Hemigaleidae).
Type-locality: Indian Ocean, South Africa.
Material examined: Two adult ♀♀ from a single host specimens collected on 23rd of February 2005 from uShaka Marine World, Durban.
Type-material: One adult ♀ (holotype) (SAMC-A096834). Remaining female (1 dissected) retained in the personal collection of the author.
ZooBank registration: To comply with the regulations set out in Article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Live Science Identifier (LSID) for Pandarus echinifer n. sp. is urn:lsid:zoobank.org:pub:B4AB53D1-D770-4BAF-8992-51D3646B8F13.
Etymology: The specific name echinifer (sea-urchin bearer) refers to the structure of the distomedial spine on the last exopodal segment of leg 1 that reminds of the spines on a sea-urchin.
Description (Figs. 10, 11, 12)
Adult female [Based on two specimens, one without abdominal plate]. Female typical Pandarus morphology. Body length from anterior margin to posterior margin of abdominal plate/abdomen 6.25–6.5 mm (mean 6.4), cephalothorax length 2.5–2.8 mm (mean 2.65), cephalothorax width 3.75–3.85 mm (mean 3.8), abdominal plate length and width 1.25 mm. Cephalothorax dorsally with uneven posteromedial margin with several sharp denticles (about 5 on either side of middle) (Fig. 10a). Dorsal thoracic plates of second somite slightly longer than that of third somite, reaching only about a third length of dorsal plate of fourth somite (Fig. 10a). Genital complex with paired posterior protrusions (Fig. 10a). Dorsal abdominal plate mostly circular with narrow anterior extension joining with genital complex (Fig. 10a). Caudal rami (Fig. 10a) lateral to dorsal abdominal plate, extending beyond middle of dorsal abdominal plate, slender, sharply pointed distally with 2 processes dorsally and 1 spine and small pinnate seta ventrally, slight basal medial expansions (Fig. 10b).
Antennule, antenna, oral cone, mandible, maxillule (Fig. 10c), maxilla and maxilliped mostly similar to congeneric members.
Legs 1–3 (Figs. 11a–d), biramous, 2-segmented, leg 4 (Fig. 11e) 1-segmented. Armature formula as follows with spines (Roman numerals) and setae (Arabic numerals):
Endopod | Exopod | |||
|---|---|---|---|---|
1 | 2 | 1 | 2 | |
Leg 1 | 0-0 | 3 | I-0 | III, I, 3 |
Leg 2 | 0-0 | 4 | I-0 | III, I, 6 |
Leg 3 | 0-0 | 2 | I-0 | III, I, 2 |
Leg 4 | 0 | - | IV, I, 1 | - |
Distomedial spine on leg 1 exopod heavily spinulated ((Figs. 11b, 12a, b). Leg 5 (Fig. 11f) small pointed tubercle and one small seta with adjacent seta.
Remarks
Females of P. echinifer n. sp. are also part of the “cranchii group” with the dorsal plates of thoracic somite two extending “well beyond” the posterior margin of the plate of somite three (Cressey 1967), although they are only slightly longer than the plate of somite three. Additionally, the plates of thoracic somite two extends only to the anterior third of the plate of somite four while the dorsal abdominal plate is longer than the exposed caudal rami. These features are very similar to those P. sinuatus. Additionally, the presence of slight basal medial expansions on the caudal rami of P. echinifer n. sp. individuals similar to those in P. sinuatus also cause difficulty distinguishing among females of P. echinifer n. sp., P. sinuatus and P. smithii. The structure of the distomedial spine on the last exopodal segment of leg 1 can be used to distinguish among the three species with similar dorsal morphology i.e. P. echinifer n. sp., P. sinuatus and P. smithii with that of P. echinifer n. sp. heavily spinulated (Figs. 11b, 12a, b), that of P. sinuatus appearing more bulbous (Figs. 8d–f, 9a) and that of P. smithii a bulbous process with a slightly extended tip, giving it a slightly bifid appearance (Figs. 6b–d).The spinules covering the tip of the distomedial spine on the last exopodal segment of leg 1 of P. echinifer n. sp. is reminiscent of that of P. cranchii (see Fig. 2c, d) but with more prominent spinules (see Fig. 12b) while the relative lengths of the dorsal thoracic plates and the lengths of the caudal rami vastly differ between P. echinifer n. sp. and P. cranchii. Furthermore P. cranchii does not have slight basal medial expansions on the caudal rami and thus the two species are easily distinguished.
Molecular analysis
The complete dataset consisted of 585 base pairs belonging to 13 Pandarus COI sequences (10 sequences downloaded from Genbank and three newly generated sequences). The uncorrected pairwise sequence divergences estimated amongst the Pandarus species range from 0% to 25% (Table 1). The interspecific sequence divergences vary between 15–25% with that between P. satyrus (PP434798, FJ447391) and P. cranchii (FJ447387, PP434799, PP434800) being 20–22%, between P. satyrus (PP434798, FJ447391) and P. smithii (FJ447388) 16% and 19% and between P. satyrus (PP434798, FJ447391) and P. sinuatus (FJ447389, FJ447390) 24 and 25%. The sequence divergence between P. cranchii (FJ447387, PP434799, PP434800) and P. smithii (FJ447388) is 22% while that between P. cranchii (FJ447387, PP434799, PP434800) and P. sinuatus (FJ447389, FJ447390) are 23 and 24%. The sequence divergence between P. smithii (FJ447388) and P. sinuatus (FJ447389, FJ447390) is 21%. Intraspecific sequence divergences vary between 0–3%. The sequence divergence between the two P. satyrus sequences (collected from I. oxyrhinchus (FJ447391) and R. typus (PP434798)) is 3% and the divergence between the two P. sinuatus sequences (both from C. taurus (FJ447389, FJ447390)) is 1%. Furthermore, there is no difference between the P. cranchii sequence collected from S. lewini (FJ447387) and those collected from R. typus (morphologically identified as P. cranchii – PP434799, PP434800) received from Ningaloo Park, Western Australia as well as between the P. cranchii sequences and the Pandarus sp. sequence (KJ551843) also from Ningaloo Reef, Western Australia. Thus, this sequence is also from P. cranchii. Interestingly, the divergences between the P. cranchii sequences (FJ447387, KJ551843, PP434799 and PP434800) and that of P. rhincodonicus (HG942363) (Austin et al. 2016) is only 3%. Thus, P. rhincodonicus is the same species as P. cranchii as indicated by a sequence divergence of 0–3%. Furthermore, the sequence divergences between P. satyrus (PP434798, FJ447391) and others identified as P. satyrus (collected from P. glauca from the Mediterranean Sea (OL457303, OL457304, OL457305)) (Palomba et al. 2022) are 15 and 17% falling within the interspecific sequence divergence range rather than the intraspecific sequence divergences. This also applies to the sequence divergences between these P. satyrus sequences (OL457303, OL457304, OL457305) and all the other sequences in the dataset implying that these sequences may belong to a completely different species.
Significant non-synonymous substitutions (where more than one sequence share the substitution) in the dataset occurred due to three transitions and one transversion. A first codon position transition is shared by all the P. cranchii sequences (FJ447387, PP434799, PP434800, KJ551843) and P. rhincodonicus (HG942363) from GTC/A to ATT at position 94 resulting in a change in the coded amino acid from Valine to Isoleucine. Pandarus satyrus (PP434798, FJ447391) and P. sinuatus (FJ447389, FJ447390) share a change in the coded amino acid from Valine to Isoleucine due to a first codon position transition from GTT/C to ATT at position 328. A transversion at the first codon position 346 from TCT/C to GCT/C resulted in a change in amino acid from Serine to Alanine in P. sinuatus (FJ447389, FJ447390) and P. smithii (FJ447388). Lastly a first codon position transition at position 502 from GTT/A to ATC resulted in a change from the amino acid Valine to Isoleusine in P. sinuatus (FJ447389, FJ447390).
Remarks
The morphological description of P. rhincodonicus mentioned distinguishing features such as the dorsal plates of thoracic somite two extending “almost to the limit of the fused plates of” thoracic somite four while the plates of thoracic somite two of P. cranchii are “considerably shorter” than those of somite four (Norman et al. 2000). However, due to the considerable variation amongst individuals this is not always the case (see Fig. 2a) and additionally, P. satyrus also have plates of thoracic somite two almost as long as those of somite four (see Fig. 3b, Fig. 1 in Cressey (1967)). Additionally, “the posterior margin of the cephalon is armed with four tubercules instead of the serrated margin” (Norman et al. 2000) is also seen in other species e.g. P. cranchii (see Izawa 2010), P. satyrus (see Fig. 3a), P. sinuatus (see Fig. 7a) and P. echinifer n. sp. (see Fig. 10a) while that of P. smithii seems more serrated (see Fig. 5a, Fig. 7a in Izawa (2010)). “The shape of the caudal rami differ as they lack the large inner lobe characteristic of P. cranchii” (Norman et al. 2000) is invalid since P. cranchii do not have caudal rami with basal medial expansions (see Figs. A, D in Izawa (2010)). Thus, no valid distinguishing morphological characteristics are found for P. rhincodonicus while the description included features applicable to both P. cranchii and P. satyrus (see Fig. 1A in Norman et al. (2000)). It is therefore suspected that P. rhincodonicus is a synonym of P. cranchii. Based on evidence from the molecular analysis which included the sequence of P. rhincodonicus, downloaded from Genbank (and others collected from R. typus at Ningaloo Park and morphologically identified as P. cranchii) indicated that the maximum sequence divergence amongst these sequences is 3% (Table 1) similar to expected intraspecific variation and thus P. rhincodonicus is a synonym of P. cranchii.
Discussion
Of the current 14 valid species of Pandarus (Walter & Boxshall 2024), P. rouxii, P. brevicaudis and P. ambiguus should be considered as species inquirenda. Additionally, P. rhincodonicus is a synonym of P. cranchii and P. echinifer n. sp. is newly described. Thus, the number of valid Pandarus species currently is 11.
The identification of Pandarus species is compromised due to different Pandarus species that share many characteristics e.g. the relative lengths of the dorsal thoracic plates as well as varying signs of pigmentation amongst individuals (Ho 1963; Cressey 1967; Kabata 1979) and the same spine and setal formulas (Cressey 1967), while different individuals of the same species exhibit variation in the setation of the limbs (Hewitt 1967). Even though the division into the “bicolor” and “cranchii” groups can still be used as a starting point, researchers should be careful as in some individuals this is not absolutely clear (see Fig. 7a) as is the presence of the basal medial expansions to distinguish P. smithii (cf. Figs. 5b, 6a, 8a, 10b). Additionally, different species are symbionts of several host species (see current study, Shiino (1954), Lewis (1966), Cressey (1967), Alvarez & Winfield (2001) and Izawa (2010)).
From the current study, it seems like the detailed structure of the distomedial spine on the last segment of the exopod of leg 1 may be a distinguishing feature amongst similar Pandarus species (cf. Figs. 2c, d, 4f, 6b–d, 8d–f, 9a, 11b, 12a, b). Even though the distomedial spine is quite small, differences can be observed using light microscopy (see Figs. 2d, 6d, 8f, 12a, b). This character may be specifically useful if used in combination with other less distinguishing features such as the relative lengths of the dorsal plates and the caudal rami.
This report constitutes the first record of P. bicolor from M. mustelus off the west coast (Atlantic Ocean) SA while it is also the first record of P. cranchii from R. typus off the west coast (Indian Ocean) Australia. Furthermore, it is the first report of P. satyrus from C. carcharias, I. oxyrinchus, C. taurus off the east coast (Indian Ocean) SA as well as from R. typus off the west coast (Atlantic Ocean) SA and the west coast (Indian Ocean) Australia. Additionally, P. smithii is for the first time reported from C. brevipinna off the east coast (Indian Ocean) SA as well as from R. typus off the west coast (Atlantic Ocean) SA.
References
Alvarez, F., & Winfield, I. (2001). New records of Dinemoura latifolia and Pandarus smithii (Copepoda, Siphonostomatoida, Pandaridae) parasitizing the shark Isurus oxyrinchus in the Gulf of Mexico. Crustaceana, 74, 501–503. https://doi.org/10.1163/156854001750243072
Austin, C.M., Tan, M.H., Lee, Y.P., Croft, L.J., Meekan, M.G., Pierce, S.J. & Gan, H.M. (2016). The complete mitogenome of the whale shark parasitic copepod Pandarus rhincodonicus Norman, Newbound & Knott (Crustacea; Siphonostomatoida; Pandaridae) – a new gene order for the Copepoda. Mitochondrial DNA, Part A, 27, 694–695. https://doi.org/10.3109/19401736.2014.913147
Bassett-Smith, P.W. (1899). A systematic description of parasitic Copepoda found on fishes, with an enumeration of the known species. Proceedings of the Zoological Society of London, 1899, 438–507.
Carus, J.V. (1885). Prodromus Faunae Mediterraneae, sive Descriptio Animalium maris Mediterranei incolarum, quam comparata silva rerum quatenus innotiut, adiectis et nominibus vulgaribus eorumque auctoribus in commodum zoologorum. Vol. I. Coelenterata, Echinodermata, Vermes, Arthropoda. Stuttgart, E. Schweizerbart. https://doi.org/10.5962/bhl.title.11523
Cressey, R.F. (1967). Revision of the family Pandaridae (Copepoda: Caligoida). Proceedings of the United States National Museum, 121, 1–113. https://doi.org/10.5479/si.00963801.121-3570.1
Dana, J.D. (1853). Crustacea. Part II. United States Exploring Expedition during the years 1838, 1839, 1840, 1841, 1842 under the command of Charles Wilkes. U. S. N. Philadelphia, Sherman. 14, 686–1618.
Dippenaar, S.M. (2004). Reported siphonostomatoid copepods parasitic on marine fishes of southern Africa. Crustaceana, 77, 1281–1328. https://doi.org/10.1163/1568540043165985
Dippenaar, S.M. (2024). Twenty years later: Biodiversity of marine symbiotic Siphonostomatoida (Copepoda) off Southern Africa. Zootaxa, 5419, 085–111. https://doi.org/10.11646/zootaxa.5419.1.3
Folmer, O., Black, N., Hoen, W., Lutz, R., & Vrijenhoek, R. (1994). DNA primers for amplification of Mitochondrial Cytochrome C Oxidase subunit 1 from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294–299.
Froese, R., & Pauly, D. (2024). FishBase. World Wide Web electronic publication. http://www.fishbase.org version (02/2024). Accessed on 12 April 2024
Hewitt, G.C. (1967). Some New Zealand parasitic Copepoda of the family Pandaridae. New Zealand Journal of Marine and Freshwater Research, 1, 180–264. https://doi.org/10.1080/00288330.1967.9515202
Ho, J.-S. (1963). On five species of Formosan parasitic copepods belonging to the suborder Caligoida. Crustaceana, 5, 81–98. https://doi.org/10.1163/156854063x00363
Humes, A.G., & Gooding, R.U. (1964). A method for studying the external anatomy of copepods. Crustaceana, 6, 238–240. https://doi.org/10.1163/156854064x00650
ICZN (2012). International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bulletin of Zoological Nomenclature, 69, 161–169.
Izawa, K. (2010). Redescription of eight species of parasitic copepods (Siphonostomatoida, Pandaridae) infecting Japanese elasmobranchs. Crustaceana, 83, 313–341. https://doi.org/10.1163/001121609x12591347509329
Kabata, Z. (1979). Parasitic Copepoda of British Fishes. London: The Ray Society.
Kirtisinghe, P. (1950). Parasitic copepods of fish from Ceylon III. Parasitology, 40, 77–86. https://doi.org/10.1017/s0031182000017893
Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874. https://doi.org/10.1093/molbev/msw054
Lewis, A.G. (1966). Copepod crustaceans parasitic on elasmobranch fishes of the Hawaiian Islands. Proceedings of the United States National Museum, 118, 57–154. https://doi.org/10.5479/si.00963801.118-3524.57
Maddison, D.R. & Maddison, W.P. (2001). MacClade version 4: analysis of phylogeny and character evolution. Massachusetts: Sinauer Associates.
Norman, B.M., Newbound, D.R., & Knott, B. (2000). A new species of Pandaridae (Copepoda), from the whale shark Rhincodon typus (Smith). Journal of Natural History, 34, 355–366. https://doi.org/10.1080/002229300299534
Öktener, A., Ventura, D. & Şirin, M. (2020). Occurrence of Pandarus bicolor (Siphonostomatoida: Pandaridae) on vulnerable shark species: Oxynotus centrina and Squalus acanthias from Turkish marine waters. Vie et Millieu – Life and Environment 70, 19–31.
Palomba, M., Insacco, G., Zava, B., & Santoro, M. (2022). Occurrence and molecular characterization of some parasitic copepods (Siphonostomatoida: Pandaridae) on pelagic sharks in the Mediterranean Sea. Frontiers in Marine Science, 8, 778034. https://doi.org/10.3389/fmars.2021.778034
Rangnekar, P.G. & Rangnekar, M.P. (1972). Copepods parasitic on the fishes of Bombay Family Pandaridae – I. Journal of the University of Bombay, XLI, 77–87.
Scott, T. & Scott, A. (1913). The British Parasitic Copepoda: Copepoda Parasitic on Fishes. London: The Ray Society.
Shiino, S.M. (1954). Copepods parasitic on Japanese fishes 5. Five species of the family Pandaridae. Prefectural University of Mie, 1, 291–332.
Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F., & Higgins, D.G. (1997). The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25, 4876–4882. https://doi.org/10.1093/nar/25.24.4876
Walter, T.C., Boxshall, G. (2024). World of Copepods Database. Siphonostomatoida. Accessed at: https://www.marinespecies.org/copepoda/aphia.php?p=taxdetails&id=1104 on 2024-03-04
Wilson, C.B. (1907). North American parasitic copepods belonging to the family Caligidae. Parts 3 and 4. A revision of the Pandarinae and the Cecropinae. Proceedings of the United States National Museum, 33(1573), 223–490. https://doi.org/10.5479/si.00963801.33-1573.223
Acknowledgements
Firstly, I want to acknowledge the National Research Foundation (NRF) for previous incentive funding that allowed field trips. However, any opinion, findings and conclusions or recommendations expressed in this manuscript are those of the author and therefore the NRF does not accept any liability in regard thereto. I want to thank the University of Limpopo (UL), the KwaZulu-Natal Sharks Board and Marine Dynamics for laboratory support. Additionally, I also thank D Vaughan (now Central Queensland University) and Mark Meekan (The University of Western Australia) for sending me collected specimens as well as the Ocearch Expedition (SA) that collected specimens from white sharks. The Department of Agriculture, Forestry and Fishery (DAFF) is acknowledged for permission to participate on demersal surveys and thus also the officers and crew of the FRS Africana. A Jordaan (NWU) and E van Wilpe (UP) are thanked for assistance with SEM. Furthermore, I thank T Mangena (UL) for assistance with molecular work and BP Jordaan (then UL), MC Lebepe (UL) and T Mangena (UL) with assistance during collection trips.
Funding
Open access funding provided by University of Limpopo. None.
Author information
Authors and Affiliations
Contributions
SMD conceived and designed the research, wrote the manuscript, did the illustrations and analyse and interpret the molecular data.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she has no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dippenaar, S.M. Pandarus Leach, 1816 (Copepoda: Siphonostomatoida: Pandaridae) species collected from elasmobranchs off South Africa with the description of Pandarus echinifer n. sp.. Syst Parasitol 101, 46 (2024). https://doi.org/10.1007/s11230-024-10167-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11230-024-10167-y