Abstract
The genus Myzobdella groups five species of leeches parasites of fishes mainly of freshwater but with tolerance to brackish waters. Native distribution of these species includes the New World from North to South America. Myzobdella lugubris Leidy, 1851, the type species of the genus, was briefly described based on specimens from the USA, but subsequently their morphology, known distribution and host range were expanded; however, less is known about the other four species of the genus. As part of a survey focusing on characterizing the diversity of leeches from Mexico, specimens of Myzobdella patzcuarensis (Caballero, 1940), from the type locality of the species were included for the first time in a phylogenetic study. In addition, specimens assigned to Myzobdella from the southeast of Mexico as well as from Nicaragua, were also included. In the resulting phylogenetic tree, our newly generated sequences were found nested in the same clade that M. lugubris; with unresolved relationships and relatively low genetic divergence, suggesting conspecificity. In addition, the internal morphology of the specimens of Myzobdella from Mexico is consistent with the description of M. lugubris. Our morphological examination reveals high degrees of variability in the external pigmentation of the specimens. Based on our results we formally synonymize M. patzcuarensis under M. lugubris.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Piscicolidae Johnston 1865 (Annelida: Hirudinida) is a family of parasitic leeches mainly associated with fishes, with about 170 described species around the world, two-thirds of which are marine species (Magalhães et al., 2021). Investigation about the systematics of this group is scarce in comparison with their freshwater counterparts, and the classification within the family remains unresolved (Williams & Burreson, 2006). Historically, three subfamilies were recognized: Platybdellinae Epshtein 1970, Piscicolinae Johnston 1865 and Pontobdellinae Llewellyn 1966. These subfamilies were proposed in the basis on the absence or presence of a single, or two pairs of pulsatile vesicles, respectively. So far, only members of Pontobdellinae have been recovered as a monophyletic group in phylogenetic studies (Williams & Burreson, 2006; Utevsky & Utevsky, 2018).
The genus Myzobdella was erected by Leidy, 1851 based on the study of specimens found attached to crabs of the genus Callinectes Stimpson (Decapoda: Portunidae) from an undefined locality, but must likely from Philadelphia, USA. The description of M. lugubris, the type species of the genus, is brief and provides no information about its geographic distribution or the designation of the holotype or paratypes; however, it was thought to be a brackish species (Leidy & Cassin, 1850). Meyer (1940) recognized the genus Illinobdella to include four freshwater fish-leech species from North America: Illinobdella alba Meyer, 1940, I. elongata Meyer, 1940, I. moorei Meyer, 1940 and I. richardsonii Meyer, 1940 and differentiated the new species from M. lugubris based on their habitat and host association, since all Illinobdella species are typically found in freshwater environments parasitizing fishes. In the same year, a morphologically similar species was described in Mexico: Illinobdella patzcuarensis; parasite of the charal fish Chirostoma estor Jordan (Teleostei: Atherinopsidae) from Patzcuaro Lake, Michoacán.
Further studies on Myzobdella lugubris have established that this species has a broad geographic distribution along USA and Canada, dwelling brackish and freshwater habitats, and interestingly, able to parasitize both crustaceans and fish (see Moore, 1946; Hutton & Sogandares-Bernal, 1959; Klemm, 1972; Daniels & Sawyer, 1975). Sawyer et al. (1975) and Daniels & Sawyer (1975) found that neither habitat nor host preference are reliable features for the separation of species of Illinobdella and M. lugubris. Also, such studies found no differences in internal and external anatomical traits between species, therefore all Illinobdella species from USA were transferred to Myzobdella and synonymized with Myzobdella lugubris, with the notable exception of M. patzcuarensis. Currently, in addition to M. lugubris and M. patzcuarensis, three species are considered valid within the genus: Myzobdella reducta (=Piscicolaria reducta Meyer, 1940), from USA and Myzobdella uruguayensis Mañé-Garzón and Montero, 1977 and Myzobdella platensis (Cordero, 1933) from South America (Uruguay, Argentina and Brazil). Previous phylogenetic analysis of Piscicolidae included only M. lugubris and M. reducta (Williams & Burreson, 2006), showing low genetic variation between both species; hence, Saglam et al. (2018) suggested that both specimens might belong to M. lugubris, so the remaining species are unrepresented.
Records of Myzobdella lugubris have been reported in Hawaii and Italy (Williams & Burreson, 2006; Liuzzo et al., 2018), where they were likely accidentally introduced (Lages et al., 2021). More recently, Myzobdella sp. was also reported in Nicaragua, parasitizing freshwater cichlids (Santacruz et al., 2022). Myzobdella species are important for the economy due to the damage they may cause to fish (Appy & Cone, 1982; Faisal et al., 2011; Volonterio et al., 2004) and crab hosts (Severino-Rodrigues & de Almeida-Duarte, 2020; Zara et al., 2009); and due their potentially role as vectors of blood parasites (Faisal & Schulz, 2009).
As part of a survey to characterize the morphologic and genetic diversity of fish leeches from Mexico, we collected samples of M. patzcuarensis from its type locality (Lake Patzcauro, Mexico) and additional specimens from the states of Veracruz, Yucatán, Quintana Roo, Mexico and from Nicaragua lake, Nicaragua.
Materials and methods
Sample collection
Fish specimens of Vieja sp. were recollected in January–February 2019, and November 2021 in Escondida lagoon, Los Tuxtlas, Veracruz, Mexico (18° 35′ 25″ N, 95° 05′ 23″ W). Fish specimens were captured using cast net and transported alive to the laboratory. Fish were examined for leeches attached to their external surface under a stereomicroscope. Leeches were removed with fine brushes and placed in tap water and examined in vivo, to distinguish morphological traits. For further morphological studies, leeches were fixed in warm (near boiling) 4% formalin and preserved in 70% ethanol. For molecular analyses, three specimens were preserved in 100% ethanol. Additionally, we generated DNA sequences of three specimens from Patzcuaro, Michoacan, Mexico (19° 37′ 30″ N, 10° 37′ 31″ W), donated by A. Oceguera-Figueroa; two specimens from Sisal, Yucatán (21° 11′ 29.6″ N, 89° 56′ 53.9″ W) and Isla Tamalcab, Quintana Roo, Mexico (18° 36′ 29″ N, 88° 12′ 14″ W), donated by J. Arroyave and S. Monks respectively; and two specimens preserved in alcohol and deposited in the Colección Nacional de Helmintos (CNHE), Instituto de Biología, Universidad Nacional Autónoma de México (UNAM) under number: 7252, of Myzobdella sp. collected in Nicaragua lake, Nicaragua (11° 10′ 05.23″ N, 84° 58′ 44.08″ W) (Santacruz et al. 2022).
Morphological analyses
Four leeches were stained with Gomori’s trichrome, and nine with a mixture of Meyer’s paracarmin and haematoxylin, dehydrated through graded ethanol series, cleared in methyl salicylate, and mounted in Canada balsam. Morphometric data were obtained with a micrometric ruler adapted to a light microscope Olympus XI (Olympus, Tokyo, Japan). Measurements are presented in in mm as the mean with the range and number of individuals studied in parentheses. For Scanning electron microscopy (SEM), specimens were dehydrated through ethanol series, dried using a K850 Critical Point Drier (Emitech, Ashford, England), sputter coated with gold with Q150R modular Coating System (Ashford, England), and examined at 15 kV in a Hitachi SU1015 SEM (Hitachi, Tokyo, Japan). Specimens collected were compared with voucher specimens of Myzobdella patzcuarensis deposited at the Colección Nacional de Helmintos (CNHE), Instituto de Biología, Universidad Nacional Autónoma de México (IB-UNAM). Newly collected specimens were deposited in CNHE.
Morphological identification of specimens was based on Sawyer et al. (1975) and Saglam et al. (2018); the diagnostic traits which bear Myzobdella lugubris are: one pair of eyespots, tegument translucent, trachelosome and urosome visibly divided, two annuli separating gonopores, five pairs of testisacs and midbody somites with 12 or 14 annuli.
Molecular procedures
Tissue from the leech caudal sucker was removed and used for total DNA extraction using Invitrogen PureLink® Genomic DNA mini kit (ThermoFisher Scientific, Pittsburgh, Pennsylvania) following the manufacturer’s protocol. The next mitochondrial loci: cytochrome C oxidase subunit 1 (COI), nicotinamide adenine dinucleotide dehydrogenase subunit I (nad1), 12S rDNA and the nuclear: 18S rDNA, were amplified following Williams & Burreson (2006) and Saglam et al. (2018) protocols. PCR products were visualized by electrophoresis on an agarose gel. Successful amplifications were purified using CentriSep 96 filter plates (ThermoFisher Scientific, Pittsburgh, Pennsylvania) with Sephadex G-50 (Cytiva, Marlborough, Massachusetts). Sequencing reactions included 0.4 µl BigDye Terminator v. 3.1 (Applied Biosystems, Waltham, Massachusetts), 2 µl Buffer 5x, 4 µl ddH2O, 1 µl of primer at 10 µM, and 3 µl purified PCR product (total volume 10 µl). Samples were purified using Sephadex G-50, then 25 µl de EDTA 0.5 mM was added to each sample and finally sequenced in an ABI-PRISM 3100 (Applied Biosystems® Waltham, Massachusetts) sequencer at the Laboratorio Nacional de Biodiversidad (LANABIO), IB-UNAM. Complementary sequences were assembled and edited using Geneious ver. 5.1.7 (Biomatters Ltd., Auckland, New Zealand).
Phylogenetic analysis
Multiple sequence alignment was performed in MAFFT online version (Katoh et al., 2018) applying default parameters. Pontobdella macrothela Schmarda, 1861 was used as outgroup based on previous phylogenetic analyses (Saglam et al., 2018; Utevsky & Trontelj, 2004). Details of the sequences included in the analysis are provided in Table 1. Concatenated matrix including the four genetic markers was constructed with Mesquite v. 3.7 (Maddison & Maddison, 2023). Final dataset included 23 terminals and 3317 aligned characters. Phylogenetic analysis was performed under Maximum Likelihood approach in IQ-Tree (Nguyen et al., 2015) using the concatenated dataset, with evolutionary model found with ModelFinder (Kalyaanamoorthy et al., 2017) indicating each partition in a file apart, called with -p function and with rapid Bootstrap option (Hoang et al., 2017) with 1000 pseudo-replicates. Best model fit to cox1 was TIM+F+G4, to nad1 K3Pu+F+G4, to 12S K3Pu+F and to 18S was TNe+I. We trimmed cox1 dataset to 576 bp to minimize alignment gaps and calculated pairwise genetic distances with Mesquite v. 3.7 under the K2P substitution model, as is suggested by Nei & Kumar (2000).
Results
Phylogeny
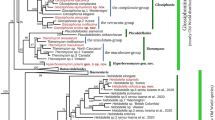
In the resulting phylogenetic tree, the genus Myzobdella is recovered as a monophyletic group with high node support (Fig. 1a). The relationships between samples of M. lugubris, M. patzcuarensis and Myzobdella sp. from Yucatán and Veracruz and Nicaragua are unresolved with marginal branch lengths, and nodes poorly supported. There was no noticeable phylogenetic separation between samples from Mexico and Central America with those from the USA and Italy.
a Maximum likelihood tree based on concatenate dataset, showing the phylogenetic position of Myzobdella patzcuarensis sequences from the type locality in bold, new sequences signalized with an asterisk. b Drawing of internal anatomy of Myzobdella lugubris collected in Veracruz, Mexico CNHE: 11660. (e) Eyespot; (p) proboscis; (sg) salivary glands; (agc) accessory gland cells; (a) atrium; (o) ovisac; (t) testisacs; (cg) cocoon glands; (c) crop; (pc) post caeca. Scale bar 1 mm.
The cox1 mean genetic distance between M. patzcuarensis from its type locality and Myzobdella sp. from Veracruz, Yucatán and Quintana Roo, Mexico is 1.16% (0 to 2.47) and between M. patzcuarensis and Myzobdella sp. from Nicaragua is 0.52% (0.52). Instead, between M. patzcuarensis and M. lugubris from USA is 1.4 (0.69 to 2.47). Myzobdella lugubris from the USA and Italy exhibit interpopulation divergences ranging from 0.17% to 2.86% and new collected samples fit in this interval. The sample from Quintana Roo is nested with M. lugubris from VA, USA (1) and MD, USA (1, 2) (Fig. 1a). This sample has pairwise genetic distances that do not surpass 2.7% with respect to the remaining samples from Mexico and from Nicaragua. The mean genetic distances within Myzobdella clade are 1.35% (0 to 3.01).
Morphology
Family Piscicolidae Johnston, 1865
Myzobdella Leidy, 1851
Myzobdella lugubris Leidy, 1851
Myzobdella patzcuarensis (Caballero, 1940) n. syn.
Zoobank identifier: urn:lsid:zoobank.org:act:6B4B6675-A0CF-4FD7-9F7A-FF49B9E8E154
Other synonyms: Ichthyobdella funduli Verrill, 1872; Piscicola funduli Pratt, 1935; Illinobdella alba Meyer, 1940; I. elongata Meyer, 1940; I. richardsoni Meyer, 1940; I. moorei Meyer, 1940; Myzobdella lubrigis Pearse, 1948; M. funduli Moore, 1952; M. moorei Meyer & Moore, 1954; Ichthyobdella rapax Wass, 1972.
Collection material from CNHE: Myzobdella patzcuarensis from its type locality Patzcuaro, Michoacán, five specimens (CNHE: 1684, 1692, 1684) ex. Algansea lacustris Steindachner; two specimens (CNHE:1687) ex. Chirostoma estor; two specimens (CNHE: 1688, 1690) ex. Goodea atripinnis Jordan. Myzobdella patzcuarensis from Infiernillo, Michoacán, one specimen (CNHE: 673) ex. Oreochromis aureus (Steindachner) and one (CNHE: 1699) ex Coptodon zillii (Gervais), one specimen (CNHE: 1700) ex. Ictalurus balsanus (Jordan et Snyder). Myzobdella sp. two specimens (CNHE: 7252) from Lake Nicaragua, ex Parachromis sp., caudal sucker processed to molecular analyses.
Newly collected samples: 25 specimens from Los Tuxtlas, Veracruz parasitizing the pectoral fin of Vieja sp. Nine stained individuals (CNHE: 11660); two individuals SEM processed, three individuals aimed to molecular analyses and 11 alcohol preserved specimens (CNHE: 11661).
Additional material observed: paratype of Illinobdella alba from Illinois, USA ex. Ictalurus punctatus (Rafinesque) collected by M. C., Meyer, 1936 (CNHE: 1702).
External morphology. Body elongated and smooth, 10.2 total length (4–20, n = 24), 1.6 of maximum width (0.8–3, n = 24); divided in two portions, trachelosome and urosome. Integument translucent. Anterior sucker small, semi-circular 0.6 length (0.40–0.85, n = 24) and 0.7 wide (0.45–0.8, n = 24). Nuchal constriction separating anterior sucker present. Mouth pore small, located in the center of the anterior sucker. Eyespots visible, one pair at somite III. Dorsal surface of the trachelosome with seven transversal stripes, first stripe beginning anterior to the eyespots and the last just before urosome (Figs. 1b, 2b, c). Constriction separating trachelosome and urosome conspicuous. Urosome with three to four longitudinal stripes. Gonopores on the ventral surface of the urosome, separated by two annuli, XI a2 and XI a3 a1 (Fig. 2a). Midbody somites with 14 annuli. Nephridiopores of the trachelosome not seen. Eleven pairs of nephridiopores visible at ventral surface of urosome, situated on lateral margin of the ventral surface (Fig. 2a). Posterior sucker smaller than the maximum body wide 0.98 length (0.58–1.2, n=24) and 1 wide (0.59–1.9, n = 24).
Internal morphology. Pharynx short, reaching somite X. Salivary glands abundant and diffused in parenchyma in somites IX to X or reaching XI, apparently with conducts opening to the base of proboscis. Bacteriomes not seen. Crop caeca with five pairs of globular chambers, located between testisacs. Post caecum saccular, almost reaching posterior end of body. Intestinal caeca not seen. Male reproductive system consisting in five pairs of testisacs. Testisacs large and oval, intersegmentally arranged. Accessory gland cells present, compact, anterior to atrial cornua. Atrial cornua voluminous, tubular not coiled, embracing until 2/3 of trachelosome maximum wide. Deferent conducts coiled. Female reproductive system consisting in a pair of ovisacs, tubular, long and s-shaped, reaching the first pair of testisacs. Cocoon glands lateral, with a longitudinal path, from clitellar area to level of the post-caecum.
Remarks
Caballero (1940) described Illinobdella patzcuarensis based on specimens from Lake Pátzcuaro, Michoacán. In spite of clearly recognizing the similarities between the new species and Illiobdella alba, described from the USA, the author highlighted some morphological differences of the Mexican forms, such as the presence of 14 pairs of nephridiopores and differences in the shape of the atrium (not specified) and mainly the presence of “reserve cells” in M. patzcuarensis. Based on the specimens studied here, “reserve cells” located along the proboscis (Fig. 1b) correspond to salivary glands, as described by Meyer in the descriptions of Illiobdella spp. Caballero found similar “reserve cells” along at the intestinal portion of body, reinforcing his idea of storage function. In the specimens studied here, these cells were found forming an antero-posterior path, laterally in the body. This type of cells were mentioned by Meyer in the description of I. elongata and I. moorei but he thought they were related with cocoon production. Probably this is the correct function of these cells, which are also present in other piscicolids and their occurrence depends on reproductive stage of the leech (Sawyer, 1986). Both M. lugubris from USA and those Myzobdella samples from Mexico have salivary glands of varying in size and some individuals present developed cocoon glands, whence, it is not a distinctive character between these taxa.
In the specimens studied here, 11 pairs of nephridial pores restricted to the urosome were observed; this number is consistent with description I. alba of Meyer (1940). In the transversal sections of M. patzcuarensis analyzed by Caballero, he found fourteen pairs of nephridiopores in the whole body (trachelosome and urosome). Thus, it is possible that the observations of Meyer (1940) were based only on the urosome (as in this study), and Caballero (1940) observed the remaining three pores situated at trachelosome. Three nephridial pores at trachelosome and eleven at urosome is consistent with the fourteen nephridial pores seen in Rhynchobdellida (see Sawyer, 1986, Vol. 1, Fig. 4.2).
Based on the current morphological evidence, no significant differences were found between M. patzcuarensis and M. lugubris. Therefore, we formally synonymize the former with M. lugubris – the valid name for the Mexican forms. Regarding the remaining internal traits, specimens assigned to Myzobdella lugubris from all known populations, both previously published, and this study have similar internal configuration. However, some differences in the external morphology between Mexican specimens and those from USA and Italy are remarkable. In the case of individuals from Patzcuaro, the body coloration has been reported as completely white (Caballero, 1940), while living individuals from Los Tuxtlas display black coloration with dark-brown to black stripes (Fig. 2b). The stripes in specimens from Los Tuxtlas differ from those of M. lugubris from Philadelphia, which were described as zig-zagged longitudinal stripes (Saglam et al., 2018), versus straight stripes in material examined here (Fig. 2b). This highlights the great variability in external pigmentation.
Myzobdella lugubris can clearly be differentiated from M. platensis and M. uruguayensis but not so from M. reducta. Myzobdella lugubris has a posterior sucker less width than body width, while in M. platensis posterior sucker is up to twice wider than body; M. lugubris has a pair of eyespots while M. uruguayensis presents two pairs. In the original description of M. reducta (=Piscicolaria reducta), mid-body somites are reported with three annuli and a total of eleven pairs of nephridiopores along the body, such characters were used to distinguish M. reducta from other species (Meyer, 1940); however, this number of annuli per somite might be wrong given that the most common condition for the genus is 14 annuli per complete somite (Moore, 1946; Sawyer, 1986; Mañé-Garzón & Montero, 1977), and common in other Piscicolidae. For example, in Austrobdella, the sister group of Myzobdella (Williams & Burreson, 2006; Utevsky et al., 2021) has six annuli per somite (Burreson, 1977; Curran et al., 2016). Body coloration has been suggested as diagnostic character to differentiate between M. lugubris and M. reducta, however, given the variation in body coloration, the separation between both and the validity of the last mentioned requires further analysis.
Moore (1938) recorded a leech morphologically similar to Myzobdella platense (=Piscicola platense) parasitizing Rhamdia guatemalensis in a cave (cenote) in Yucatán, México. Its identification was based mainly on the characteristics of the clitellum, however, the size of posterior sucker (1 mm), with respect to maximum width of body (1.2 mm) resembles M. lugubris instead of M. platensis (see above). In addition, the specimens were poorly preserved, so the identification is doubtful (Moore, 1938). In our phylogenetic tree, the leech specimen from Yucatán, was found nested within the clade comprising M. lugubris sequences, suggesting that samples from the area may be conspecific with M. lugubris.
Discussion
Myzobdella patzcuarensis, as well as their host, Chirostoma estor (‘pescado blanco’) have been considered part of the representative fauna of Lake Patzcuaro, Mexico (Oceguera-Figueroa & León-Règagnon, 2014; Torres, 1993). According to the new data presented here, M. patzcuarensis seems to be a junior synonym of M. lugubris instead of representing an independent species. Historical records indicate that in 1930, the largemouth bass Micropterus salmoides (Lacépède) was introduced to Lake Patzcuaro with fishery purposes (Solorzano, 1955), and likely M. lugubris was co-introduced.
Largemouth bass were also introduced to Nicaragua between 1950 and 1960 but their populations disappeared years later (Heidinger, 1976); the introduction of M. lugubris could have been co-introduced in the area and then colonized native fish. Alternatively, the introduction of this leech could be recent since this species has been recorded only in a single Parachromis sp. individual from Lake Nicaragua (Santacruz et al., 2022). Additional records of introduced leeches in South and Central America have been published before, M. lugubris has been reported in Venezuela (Heidinger, 1976) and more recently, Oceguera-Figueroa & Pacheco-Chaves (2012) recorded the Asian leech Barbronia weberi in Costa Rica and Kvist et al. (2018) recorded Placobdella parasitica in Panama.
Based on the present work, with the synonymization of M. patzcuarensis with Myzobdella lugubris now the species comprises 11 junior synonyms. Sawyer et al. (1975) included Cystobranchus virginicus as a junior synonym of M. lugubris based on a sample collected by Paperna & Zwerner (1974) York River, Virginia, USA. That sample resembles morphologically to Myzobdella lugubris, hence it could have been only a misidentification of samples by Paperna & Zwerner (1974). Cystobranchus virginicus sensu stricto has distinctive characters that separate it from Myzobdella species as distinct annulation (7 instead 14), testes in six pairs (instead five pairs) and present pulsatile vesicles (instead body surface smooth) (Hoffman, 1964). Thus, in our opinion, C. virginicus is a valid species, not junior synonyms of M. lugubris.
Conclusions
External traits such as body color and number and shape of dorsal lines are a highly variable in M. lugubris. These, together with the broad salinity spectrum of habitat and the low host specificity, have confused taxonomists who described additional species repeatedly. Using molecular technics, especially comparison of DNA “barcoding” region, we identified all the samples as members of the same species, which seems to be widely introduced in Mexico, Central America and Europe. Our study highlights the need to include molecular data in order to evaluate morphological variation, together with a wide geographical sampling across the species distribution range.
Data availability
Voucher specimens were deposited in the Coleccción Nacional de Helmintos, Instituto de Biología, Universidad Nacional Autónoma de Mexico, Mexico City (collection numbers: 11660 and 11661). Newly generated DNA sequences are available in GenBank database at https://www.ncbi.nlm.nih.gov/genbank/ reference numbers indicated in Table 1.
References
Apakupakul, K., Siddall, M. E., & Burreson, E. M. (1999). Higher level relationships of leeches (Annelida: Clitellata: Euhirudinea) based on morphology and gene sequences. Molecular Phylogenetics and Evolution, 12(3), 350–359.
Appy, R. G., & Cone, D. K. (1982). Attachment of Myzobdella lugubris (Hirudinea: Piscicolidae) to logperch, Percina caprodes, and brown bullhead, Ictalurus nebulosus. Transactions of the American Microscopical Society, 101, 135–141.
Burreson, E. M. (1977). A New Marine Leech Austrobdella californiana n. sp. (Hirudinea: Piscicolidae) from Southern California flatfishes. Transactions of the American Microscopical Society, 96, 263–267.
Caballero, C. E. (1940). Sanguijuelas del lago de Pátzcuaro y descripción de una nueva especie, Ilinobdella patzcuarensis. XIV. Anales del Instituto de Biología, Universidad Nacional Autónoma de México, 11, 449–464.
Curran, S. S., Phillips, A. J., Overstreet, R. M., Benz, G. W., & Henningsen, A. D. (2016). Austrobdella cairae n. sp., an Oioxenous Marine Leech (Clitellata: Piscicolidae) from the Banded Guitarfish, Zapteryx exasperata, in the Northeastern Pacific Ocean. Journal of Parasitology, 102, 179–186. https://doi.org/10.1645/15-829
Daniels, B. A., & Sawyer, R. T. (1975). The biology of the leech Myzobdella lugubris infesting blue crabs and catfish. The Biological Bulletin, 148, 193–198. https://doi.org/10.2307/1540542
Faisal, M., & Schulz, C. A. (2009). Detection of Viral Hemorrhagic Septicemia virus (VHSV) from the leech Myzobdella lugubris Leidy, 1851. Parasites & Vectors, 2, 45. https://doi.org/10.1186/1756-3305-2-45
Faisal M., Schulz C., & Whelan G. (2011). High prevalence of buccal ulcerations in largemouth bass, Micropterus salmoides (Centrarchidae) from Michigan inland lakes associated with Myzobdella lugubris Leidy 1851 (Annelida: Hirudinea). Parasite: Journal de la Société Francaise de Parasitologie, 18, 79–84.
Heidinger R. C. (1976). Synopsis of biological data on the largemouth bass Micropterus almoides (Lacépède) 1802. Food and Agriculture organization of the United Nations, Fisheries Synopses, 15. Rome: Food and Agriculture organization of the United Nations.
Hoang, T. D., Chernomor, O., von Haeseler, A., Quang Minh, B., Sy Vinh, L., & Rosenberg, M. S. (2017). UFBoot2: Improving the Ultrafast Bootstrap Approximation. Molecular Biology and Evolution, 35(2), 518–522. https://doi.org/10.5281/zenodo.854445
Hoffman, R. L. (1964). A New Species of Cystobranchus from Southwestern Virginia (Hirudinea: Piscicolidae). The American Midland Naturalist, 72(2), 390–395.
Hutton, R. F., & Sogandares-Bernal, F. (1959). Notes on the Distribution of the Leech, Mysobdella lugubris Leidy, and Its Association with Mortality of the Blue Crab, Callinectes sapidus. The Journal of Parasitology, 45, 384–430.
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A., & Jermiin, L. S. (2017). ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods, 14(6), 587–589. https://doi.org/10.1038/nmeth.4285
Katoh, K., Rozewicki, J., & Yamada, K. D. (2018). MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 20(4), 1160–1166. https://doi.org/10.1093/bib/bbx108
Kvist, S., de Carle, D., Cornejo, A., & Oceguera-Figueroa, A. (2018). Biological introductions or native ranges: two curious cases of new distributional records in the Panama Canal. BioInvasions Record, 7(3), 237–244.
Klemm D. J. 1972. Freshwater Leeches (Annelida: Hirudinea) of North America. Water Pollution Control Research Series 18050 ELD05/72, USEPA.
Lages, A., de Araújo Costa, D., Gomes, N., & Antunes, C. (2021). Exotic Pumpkinseed Sunfish Lepomis gibbosus (Linnaeus, 1758) in the International Minho River (Iberian Peninsula), and Parasitic Association with Myzobdella lugubris Leidy, 1851 (Annelida, Hirudinea). Oceanography & Fisheries Open Access Journal, 13(5), 555872. https://doi.org/10.19080/ofoaj.2021.13.555872
Leidy, J., & Cassin, J. (1850). August 26th; Helminthological Contributions; No. 3; Descriptions of New Species of Birds of the Family Laniadæ, Specimens of Which Are in the Collection of the Academy of Natural Sciences of Philadelphia. Proceedings of the Academy of Natural Sciences of Philadelphia, 5, 239–254.
Light, J. E., & Siddall, M. E. (1999). Phylogeny of the leech family Glossiphoniidae based on mitochondrial gene sequences and morphological data. The Journal of Parasitology, 85, 815–823.
Liuzzo, M., Alfonso, G., Beli, E., Arculeo, M., & Marrone, F. (2018). First record of the alien leech Myzobdella lugubris Leidy, 1851 (Hirudinea, Piscicolidae) in the Palearctic. Limnetica, 37(2), 311–318. https://doi.org/10.23818/limn.37.25
Maddison, W. P., & Maddison, D. R. (2023). Mesquite: a modular system for evolutionary analysis. Version 3.81. Available at: http://www.mesquiteproject.org
Magalhães, W. F., Hutchings, P., Oceguera-Figueroa, A., Martin, P., Schmelz, R. M., Wetzel, M. J., Wiklund, H., Maciolek, N. J., Kawauchi, G. Y., & Williams, J. D. (2021). Segmented worms (Phylum Annelida): A celebration of twenty years of progress through Zootaxa and call for action on the taxonomic work that remains. Zootaxa, 4979 (1), 190–211. https://doi.org/10.11646/zootaxa.4979.1.18
Mañé-Garzón, F., & Montero, R. (1977). Myzobdella uruguayensis n. sp. (Hirudinea Piscicolidae) parasita de las branquias del bagre amarillo Rhambdia sapo (Vall.). Revista de Biologia de Uruguay, 5, 59–65.
Meyer, M. C. (1940). A Revision of the Leeches (Piscicolidae) Living on Fresh-Water Fishes of North America. Transactions of the American Microscopical Society, 59(3), 354–376.
Moore, J. P. (1938). Leeches (Hirudinea) from Yucatan caves. Carnegie Institution of Washington, 491, 67–70.
Moore, J. P. (1946). The anatomy and systematic position of Myzobdella lugubris Leidy (Hirudinea). Notulae Naturae: Academy of Natural Sciences of Philadelphia, 184, 1–12.
Nei, M., & Kumar, S. (2000). Molecular evolution and phylogenetics. Oxford: University Press.
Nguyen, L. T., Schmidt, H. A., von Haeseler, A., & Minh, B. Q. (2015). IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution, 32(1), 268–274. https://doi.org/10.1093/molbev/msu300
Oceguera-Figueroa, A., & León-Règagnon, V. (2014). Biodiversity of leeches (Annelida: Euhirudinea) in Mexico. Revista Mexicana de Biodiversidad, 85, S177–S189. https://doi.org/10.7550/rmb.33212
Oceguera-Figueroa, A., & Pacheco-Chaves, B. (2012). Registros de sanguijuelas de Costa Rica y clave para la identificación de las especies con redescripción de Cylicobdella costaricae. Revista Mexicana de Biodiversidad, 83(4), 946–957.
Paperna, I., & Zwerner, D. E. (1974). Massive leech infestation on a white catfish (Ictalurus catus): a histopathological consideration. Proceedings of the Helminthological Society of Washington, 41(1), 64–67.
Saglam, N., Saunders, R., Lang, S. A., & Shain, D. H. (2018). Phylogeny and cocoon production in the parasitic leech Myzobdella lugubris Leidy, 1851 (Hirudinidae, Piscicolidae). Acta Parasitologica, 63(1), 15–26. https://doi.org/10.1515/ap-2018-0002
Santacruz, A., Barluenga, M., & Pérez-Ponce de León, G. (2022). The macroparasite fauna of cichlid fish from Nicaraguan lakes, a model system for understanding host–parasite diversification and speciation. Scientific Reports, 12(1), 3944. https://doi.org/10.1038/s41598-022-07647-w
Sawyer, R. T. (1986). Leech Biology and Behavior (1st ed., Vol. I–III). Oxford: Clarendon Press.
Sawyer, R. T., Lawler, A. R., & Oversrteet, R. M. (1975). Marine leeches of the eastern United States and the Gulf of Mexico with a key to the species. Journal of Natural History, 9(6), 633–667. https://doi.org/10.1080/00222937500770531
Severino-Rodrigues, E., & de Almeida-Duarte, A. (2020). Environmental conditions influencing the leech infestation by Myzobdella platensis (Hirudinea: Piscicolidae) on the swimming crabs (Callinectes spp.) (Decapoda: Portunidae) in a Brazilian tropical estuary. Pan-American Journal of Aquatic Sciences, 15(2), 121–132.
Siddall, M. E., & Burreson, E. M. (1998). Phylogeny of leeches (Hirudinea) based on mitochondrial cytochrome c oxidase subunit I. Molecular Phylogenetics and Evolution, 9(1), 156–162.
Solorzano, A. (1955). La Pesca en el Lago de Pátzcuaro, Michoacán y su importancia económica regional. Ciudad de Mexico, Secretaría de Marina, Dirección General de Pesca.
Torres, A. C. (1993). Lake Patzcuaro, Mexico: Watershed and water quality deterioration in a tropical high-altitude Latin American lake. Lake and Reservoir Management, 8(1), 37–47. https://doi.org/10.1080/07438149309354457
Utevsky, S. Y., & Trontelj, P. (2004). Phylogenetic relationships of fish leeches (Hirudinea, Piscicolidae) based on mitochondrial DNA sequences and morphological data. Zoologica Scripta, 33(4), 375–385. https://doi.org/10.1111/j.0300-3256.2004.00156.x
Utevsky, A., Solod, R., & Utevsky, S. (2021). A new deep-sea fish leech of the bipolar genus Pterobdellina stat. rev. (Hirudinea: Piscicolidae) parasitic on the Antarctic toothfish Dissostichus mawsoni (Perciformes: Nototheniidae). Marine Biodiversity, 51, 1–19. https://doi.org/10.1007/s12526-020-01140-1/Published
Utevsky, A., & Utevsky, S. (2018). New Antarctic deep-sea weird leech (Hirudinida: Piscicolidae): morphological features and phylogenetic relationships. Systematic Parasitology, 95, 849–861. https://doi.org/10.1007/s11230-018-9816-y
Volonterio, O., López-De León, E., & Ponce De León, R. (2004). Infestation dynamics and histopathology of two species of freshwater leeches (Hirudinea: Piscicolidae) on teleost fish from Uruguay. Comparative Parasitology, 71(1), 21–28. https://doi.org/10.1654/4087
Williams, J. I., & Burreson, E. M. (2006). Phylogeny of the fish leeches (Oligochaeta, Hirudinida, Piscicolidae) based on nuclear and mitochondrial genes and morphology. Zoologica Scripta, 35(6), 627–639. https://doi.org/10.1111/j.1463-6409.2006.00246.x
Zara, F. J., Luiz, A., Reigada, D., Felipe, L., Passero, D., & Toyama, M. H. (2009). Myzobdella platensis (Hirundinida: Piscicolidae) Is True Parasite of Blue Crabs (Crustacea: Portunidae). The Journal of Parasitology, 95(1), 124–128. https://www.jstor.org/stable/27735535?seq=1&cid=pdf-
Acknowledgements
We thank the Posgrado en Ciencias Biológicas UNAM and Consejo Nacional de Ciencia y Tecnología (CONACYT) for the support and scholarships to GT-C, YV-U and AS to A Oceguera-Figueroa provided specimens from Pátzcuaro, as well as reviewed early and final versions of the manuscript. Thanks to G. Pérez-Ponce de León for the valuable comments to this work. We thank Jairo Arroyave (Instituto de Biología, UNAM) and Scott Monks (Instituto de Ciencias Básicas e Ingenieria, Universidad Autónoma del Estado de Hidalgo) for providing specimens of Myzobdella. Special thanks to Dario Velasco for his help in the collection of specimens from Laguna Escondida, Veracruz. Thanks to Laura Márquez, Nelly López, Andrea Jiménez and Berenit Mendoza (LANABIO), for their help with molecular biology procedures and the SEM micrographs. Thanks to Luis García-Prieto and Alejandro Oceguera-Figueroa, of the CNHE for providing assistance in the loan of vouchers of M. lugubris and the deposition of newly material collected.
Funding
This project was partially funded by Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT) No. IN215722 to A. Oceguera-Figueroa and PAPIIT No. IN212621; CONACYT No. A1-S-21694 to G. Pérez-Ponce de León and CONACYT “Ciencia Básica” No. A1-S-28293 to J. Arroyave.
Author information
Authors and Affiliations
Contributions
Study design: Ana Santacruz and Gerardo Torres-Carrera. Specimens acquisition, processing, and deposition in biological collection: Yanet Velázquez-Urrieta and Gerardo Torres-Carrera. Analyzed data: Gerardo Torres-Carrera and Yanet Velázquez-Urrieta. Writing—original draft preparation: Gerardo Torres-Carrera. Writing—review and editing: All authors contributed. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors state no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Torres-Carrera, G., Velázquez-Urrieta, Y. & Santacruz, A. Not that many leech species after all: Myzobdella lugubris and Myzobdella patzcuarensis (Annelida: Hirudinida) are the same species. Syst Parasitol 101, 38 (2024). https://doi.org/10.1007/s11230-024-10160-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11230-024-10160-5