Abstract
In survival contexts, substantial literature exists on estimating optimal treatment regimes, where treatments are assigned based on personal characteristics to maximize the survival probability. These methods assume that a set of covariates is sufficient to deconfound the treatment-outcome relationship. However, this assumption can be limited in observational studies or randomized trials in which non-adherence occurs. Therefore, we propose a novel approach to estimating optimal treatment regimes when certain confounders are unobservable and a binary instrumental variable is available. Specifically, via a binary instrumental variable, we propose a semiparametric estimator for optimal treatment regimes by maximizing a Kaplan–Meier-like estimator of the survival function. Furthermore, to increase resistance to model misspecification, we construct novel doubly robust estimators. Since the estimators of the survival function are jagged, we incorporate kernel smoothing methods to improve performance. Under appropriate regularity conditions, the asymptotic properties are rigorously established. Moreover, the finite sample performance is evaluated through simulation studies. Finally, we illustrate our method using data from the National Cancer Institute’s prostate, lung, colorectal, and ovarian cancer screening trial.



Similar content being viewed by others
Data Availability
Access to the data used in the study is granted through the National Cancer Institutes with the project number PLCO-1040 and can be obtained upon approval through the following link: https://cdas.cancer.gov/plco/.
References
Angrist, J.D., Imbens, G.W., Rubin, D.B.: Identification of causal effects using instrumental variables. J. Am. Stat. Assoc. 91(434), 444–455 (1996). https://doi.org/10.1080/01621459.1996.10476902
Bai, X., Tsiatis, A.A., Lu, W., Song, R.: Optimal treatment regimes for survival endpoints using a locally-efficient doubly-robust estimator from a classification perspective. Lifetime Data Anal. 23(4), 585–604 (2017). https://doi.org/10.1007/s10985-016-9376-x
Caudill, S.B.: Practitioners corner: An advantage of the linear probability model over probit or logit. Oxford Bull. Econ. Stat. 50(4), 425–427 (1988). https://doi.org/10.1111/j.1468-0084.1988.mp50004005.x
Chen, S., Zhang, B.: Estimating and improving dynamic treatment regimes with a time-varying instrumental variable. J. R. Stat. Soc. Ser. B Stat Methodol. 85(2), 427–453 (2023). https://doi.org/10.1093/jrsssb/qkad011
Cui, Y., Tchetgen Tchetgen, E.: A semiparametric instrumental variable approach to optimal treatment regimes under endogeneity. J. Am. Stat. Assoc. 116(533), 162–173 (2020). https://doi.org/10.1080/01621459.2020.1783272
Cui, Y., Zhu, R., Kosorok, M.: Tree based weighted learning for estimating individualized treatment rules with censored data. Electron. J. Stat. 11(2), 3927–3953 (2017). https://doi.org/10.1214/17-ejs1305
Doubleday, K., Zhou, H., Fu, H., Zhou, J.: An algorithm for generating individualized treatment decision trees and random forests. J. Comput. Graph. Stat. 27(4), 849–860 (2018). https://doi.org/10.1080/10618600.2018.1451337
Geng, Y., Zhang, H.H., Lu, W.: On optimal treatment regimes selection for mean survival time. Stat. Med. 34(7), 1169–1184 (2015). https://doi.org/10.1002/sim.6397
Gill, R.D., Johansen, S.: A survey of product-integration with a view toward application in survival analysis. Ann. Stat. 18(4), 1501–1555 (1990). https://doi.org/10.1214/aos/1176347865
Gohagan, J.K., Prorok, P.C., Hayes, R.B., Kramer, B.S.: Team: The prostate, lung, colorectal and ovarian (plco) cancer screening trial of the national cancer institute: History, organization, and status. Controll. Clin. Trials 21(6), 251–272 (2000). https://doi.org/10.1016/s0197-2456(00)00097-0
Gonzalez-Manteiga, W., Cadarso-Suarez, C.: Asymptotic properties of a generalized Kaplan–Meier estimator with some applications. J. Nonparametric Stat. 4(1), 65–78 (1994). https://doi.org/10.1080/10485259408832601
Hines, O., Dukes, O., Diaz-Ordaz, K., Vansteelandt, S.: Demystifying statistical learning based on efficient influence functions. Am. Stat. 76(3), 292–304 (2022). https://doi.org/10.1080/00031305.2021.2021984
Horowitz, J.L.: Semiparametric and Nonparametric Methods in Econometrics. Springer, New York (2009)
Imbens, G.W., Angrist, J.D.: Identification and estimation of local average treatment effects. Econometrica 62(2), 467–475 (1994). https://doi.org/10.2307/2951620
Jiang, R., Lu, W., Song, R., Davidian, M.: On estimation of optimal treatment regimes for maximizing \(t\)-year survival probability. J. R. Stat. Soc. Series B Stat. Methodol. 79(4), 1165–1185 (2017). https://doi.org/10.1111/rssb.12201
Kennedy, E.H.: Semiparametric theory and empirical processes in causal inference. In: Statistical Causal Inferences and Their Applications in Public Health Research, pp. 141–167. Springer, New York (2016)
Kianian, B., Kim, J.I., Fine, J.P., Peng, L.: Causal proportional hazards estimation with a binary instrumental variable. Stat. Sin. 31(2), 673–699 (2021). https://doi.org/10.5705/ss.202019.0096
Kramer, O., Kramer, O.: Genetic Algorithms, pp. 11–19. Springer, New York (2017)
Laber, E.B., Zhao, Y.Q.: Tree-based methods for individualized treatment regimes. Biometrika 102(3), 501–514 (2015). https://doi.org/10.1093/biomet/asv028
Lee, Y., Kennedy, E.H., Mitra, N.: Doubly robust nonparametric instrumental variable estimators for survival outcomes. Biostatistics 24(2), 518–537 (2021). https://doi.org/10.1093/biostatistics/kxab036
Lin, D.Y.: On the Breslow estimator. Lifetime Data Anal. 13(4), 471–480 (2007). https://doi.org/10.1007/s10985-007-9048-y
Mack, C.D., Glynn, R.J., Brookhart, M.A., Carpenter, W.R., Meyer, A.M., Sandler, R.S., Stürmer, T.: Calendar time-specific propensity scores and comparative effectiveness research for stage iii colon cancer chemotherapy. Pharmacoepidemiol. Drug Saf. 22(8), 810–818 (2013). https://doi.org/10.1002/pds.3386
Mebane, W.R., Sekhon, J.S.: Genetic optimization using derivatives: the Rgenoud package for r. J. Stat. Softw. 42(11), 1–26 (2011). https://doi.org/10.18637/jss.v042.i11
Mi, X., Zou, F., Zhu, R.: Bagging and deep learning in optimal individualized treatment rules. Biometrics 75(2), 674–684 (2019). https://doi.org/10.1111/biom.12990
Mitchell, M.: An Introduction to Genetic Algorithms. The MIT Press, Massachusetts (1998)
Murphy, S.A.: Optimal dynamic treatment regimes. J. R. Stat. Soc. Series B Stat. Methodol. 65(2), 331–355 (2003). https://doi.org/10.1111/1467-9868.00389
Pearl, J.: Causal diagrams for empirical research. Biometrika 82(4), 669–688 (1995). https://doi.org/10.1093/BIOMET/82.4.669
Pu, H., Zhang, B.: Estimating optimal treatment rules with an instrumental variable: A partial identification learning approach. J. R. Stat. Soc. Series B Stat. Methodol. 83(2), 318–345 (2021). https://doi.org/10.1111/rssb.12413
Qiu, H., Carone, M., Sadikova, E., Petukhova, M., Kessler, R.C., Luedtke, A.: Optimal individualized decision rules using instrumental variable methods. J. Am. Stat. Assoc. 116(533), 174–191 (2021). https://doi.org/10.1080/01621459.2020.1745814
Shi, C., Fan, A., Song, R., Lu, W.: High-Dimensional a-Learning for Optimal Dynamic Treatment Regimes. Ann. Stat. 46(3), 925–957 (2018). https://doi.org/10.1214/17-aos1570
Tang, V., Boscardin, W.J., Stijacic-Cenzer, I., Lee, S.J.: Time to benefit for colorectal cancer screening: survival meta-analysis of flexible sigmoidoscopy trials. BMJ 350, 1662 (2015). https://doi.org/10.1136/bmj.h1662
Tchetgen Tchetgen, E., Walter, S., Vansteelandt, S., Martinussen, T., Glymour, M.: Instrumental variable estimation in a survival context. Epidemiology 26(3), 402–410 (2015). https://doi.org/10.1097/ede.0000000000000262
Tsiatis, A.A.: Semiparametric Theory and Missing Data. Springer, New York (2006)
Wang, Z., Louis, T.A.: Matching conditional and marginal shapes in binary random intercept models using a bridge distribution function. Biometrika 90(4), 765–775 (2003). https://doi.org/10.1093/biomet/90.4.765
Wang, L., Tchetgen Tchetgen, E.: Bounded, efficient and multiply robust estimation of average treatment effects using instrumental variables. J. R. Stat. Soc. Series B Stat. Methodol. 80(3), 531–550 (2018). https://doi.org/10.1111/rssb.12262
Wang, L., Tchetgen Tchetgen, E., Martinussen, T., Vansteelandt, S.: Instrumental variable estimation of the causal hazard ratio. Biometrics 79(2), 539–550 (2022). https://doi.org/10.1111/biom.13792
Ying, A., Xu, R., Murphy, J.: Two-stage residual inclusion for survival data and competing risks—an instrumental variable approach with application to seer-medicare linked data. Stat. Med. 38(10), 1775–1801 (2019). https://doi.org/10.1002/sim.8071
Yu Zhou, R.S., Wang, Lan, Zhao, T.: Transformation-invariant learning of optimal individualized decision rules with time-to-event outcomes. J. Am. Stat. Assoc. 118(544), 2632–2644 (2023). https://doi.org/10.1080/01621459.2022.2068420
Zhang, B., Zhang, M.: Variable selection for estimating the optimal treatment regimes in the presence of a large number of covariates. Annal. Appl. Stat. 12(4), 2335–2358 (2018). https://doi.org/10.1214/18-aoas1154
Zhang, B., Tsiatis, A.A., Laber, E.B., Davidian, M.: A robust method for estimating optimal treatment regimes. Biometrics 68(4), 1010–1018 (2012). https://doi.org/10.1111/j.1541-0420.2012.01763.x
Zhao, Y., Zeng, D., Rush, A.J., Kosorok, M.R.: Estimating individualized treatment rules using outcome weighted learning. J. Am. Stat. Assoc. 107(499), 1106–1118 (2012). https://doi.org/10.1080/01621459.2012.695674
Zhao, Y.Q., Zeng, D., Laber, E.B., Song, R., Yuan, M., Kosorok, M.R.: Doubly robust learning for estimating individualized treatment with censored data. Biometrika 102(1), 151–168 (2015). https://doi.org/10.1093/biomet/asu050
Acknowledgements
The authors would like to express their gratitude to the editor and anonymous referee for their careful reading and useful comments, which led to an improved presentation of the paper. The authors thank the National Cancer Institute (NCI) for access to NCl’s data collected by the prostate, lung, colorectal, and ovarian cancer screening trial. The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by NCI. This work was supported by Public Health & Disease Control and Prevention, Fund for Building World-Class Universities (Disciplines) of Renmin University of China (to J.Z.) and the MOE Project of Key Research Institute of Humanities and Social Sciences (22JJD910001).
Author information
Authors and Affiliations
Contributions
Junwen Xia: first author, methodology, computation and writing. Zishu Zhan: co-first author, conceptualization, methodology and writing. Jingxiao Zhang: corresponding author, conceptualization, methodology and writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendices
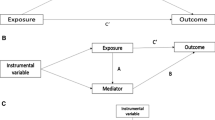
Appendix A: Illustration of the IV-related assumptions
Recall that the IV-related assumptions are assumptions A2-A7 and Fig. 1 gives an illustration of these assumptions. Among these assumptions, assumptions A2-A5 are the core assumptions for all IV-based methods and assumptions A6-A7 are required for the identifiability of the final estimand (Wang et al. 2022; Cui and Tchetgen Tchetgen 2020; Qiu et al. 2021; Wang and Tchetgen Tchetgen 2018). Assumption A2 states that the variable \(U\) is sufficient to account for the unmeasured confounding. Assumption A3 requires that the IV is associated with the treatment conditional on \({\varvec{L}}\). Assumption A4 states that the direct causal effect from \(Z\) to \(T\) is all mediated by the treatment \(A\). Assumption A5 ensures that, conditional on \({\varvec{L}}\), the causal effect of \(Z\) on \(T\) is unconfounded by \(U\) because \(U\) has no causal effect on \(Z\). Assumption A7 posits that in a model for the probability of being treated conditional on \({\varvec{L}}\) and \(U\), there exists no additive interaction between \(Z\) and \(U\). An example that satisfies this assumption is the additive probability model \(\pi (A=1|Z,{\varvec{L}},U)=g(Z,{\varvec{L}})+ h(U,{\varvec{L}})\), where \(g\) and \(h\) are measurable functions such that the range of the function \(\pi (A=1|Z,{\varvec{L}},U)\) is a subset of the interval \([0,1]\). The additive probability model can capture certain types of real-world data (Caudill 1988). Another interpretation of the assumption can be given as follows. Define the counterfactual treatment under the IV \(Z=z\) as \(A(z)\). In randomized trials where \(Z\! \perp \! \! \! \perp A(z)|(L, U)\) and \(Z\! \perp \! \! \! \perp A(z)|L\), or in observational studies under the same assumptions, the assumption A7 can be rearranged as \(E[A(1)-A(0)|{\varvec{L}}, U]=E[A(1)-A(0)|{\varvec{L}}]\). Based on the relationship between the instrument \(Z\) and the treatment \(A(Z)\), we can segment the population into four adherence type: compliers \((A(1)=1, A(0)=0)\), always-takers \((A(1)=1, A(0)=1)\), never-takers \((A(1)=0, A(0)=0)\), and defiers \((A(1)=0, A(0)=1)\) (Imbens and Angrist 1994; Angrist et al. 1996). Then assumption A7 holds if the adherence type is determined by the observed covariates \({\varvec{L}}\), such as age and disease history.
In our case, no unmeasured confounding assumption is problematic as health status is a confounder. However, the IV assumptions are more likely to be accepted. The IV relevance condition is convincing because the adherence rate is approximately 85%. This indicates a strong relationship between the screening assignment (\(Z\)) and the treatment (\(A\)). Regarding the exclusion restriction assumption, the screening assignment itself does not directly affect the health of individuals. Instead, its effect is mediated by its influence on the treatment an individual receives, which ensures the exclusion restriction assumption. Since the IV we utilized here is the randomization procedure itself, it is independent of \({\varvec{L}}, U\) and each person has the same probability \(0.5\) of being assigned to the screening group. As a result, both the IV independence and IV positivity assumptions are satisfied. To achieve the independent adherence type assumption, it is necessary to include a list of variables that can predict the adherence type in our analysis. In our case, we use age, colorectal polyps, and diabetes to characterize the non-adherence due to health status. Other important variables are listed in Sect. 5.
Appendix B: Intuition underlying the IWKME-IV
We now provide more details on the intuition underlying our estimator (2) of the counterfactual survival function. Our primary goal is to estimate the counterfactual survival function \(P(T^*(d_{\varvec{\eta }}({\varvec{L}}))> t)\) via the Kaplan–Meier-like estimator as follows, where \(\prod _{s \le t}\) is a product-based counterpart of the usual sum-based integral of calculus (Gill and Johansen 1990). It requires a consistent estimator for the differential of the counterfactual cumulative distribution function \(dP(T^*(d_{\varvec{\eta }}({\varvec{L}}))\le s)\) and another estimator for the counterfactual survival function \(P(T^*(d_{\varvec{\eta }}({\varvec{L}}))\ge s)\), where \(s\le t\).
To be concise, we illustrate the intuition behind constructing the estimator (2) with an example of constructing the estimator of the counterfactual survival function \(P(T^*(d_{\varvec{\eta }}({\varvec{L}}))\ge s)\). Let \(d_{\varvec{\eta }}({\varvec{L}})=1\), and for simplicity, omit the consideration of censoring and baseline covariates \({\varvec{L}}\). Therefore, our goal is to find a good estimator of \({P}(T^*(1)\ge s)\). Note that
where the first equality follows the independent adherence type assumption A7, the second equality is from the law of total probability, and the fourth equality follows from assumptions A2, A4, and A5. With the identification equation (4) above, we can construct \({\widehat{P}}(T^*(1)\ge s)\) mentioned below as an estimator of \({P}(T^*(1)\ge s)\). It is the same as the denominator part of our estimator (2) when there is no censoring, the baseline covariates \({\varvec{L}}\) are omitted, and \(d_{\varvec{\eta }}({\varvec{L}})=1\).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Junwen, X., Zishu, Z. & Jingxiao, Z. Doubly robust estimation of optimal treatment regimes for survival data using an instrumental variable. Stat Comput 34, 96 (2024). https://doi.org/10.1007/s11222-024-10407-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11222-024-10407-7