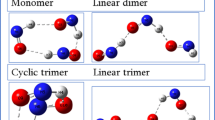
The Raman, infrared, and visible/ultraviolet spectra of stable nitrogen clusters N4 and N6 are investigated within the framework of the density functional theory and the time-dependent density functional theory. Possible configurations of dimers in which two clusters are connected by the van der Waals forces or covalent bonds by means of molecular bridges are considered. It has been found that the most effective bridges are ions of light Mg and Be metals: the energy of their binding with nitrogen clusters lies in the range 2.67–4.74 eV. The spectra of dimers have been calculated, which makes it possible to detect their formation by spectrometric methods.
Similar content being viewed by others
References
B. M. Gimarc and M. Zhao, Coord. Chem. Rev., 158, 385–412 (1997).
K. M. Dunn and K. Morokuma, J. Chem. Phys., 102, No. 12, 4904 (1995).
R. Engelke and J. R. Stine, J. Phys. Chem., 94, No. 15, 5689–5694 (1990).
M. L. Leininger, C. D. Sherrill, and H. F. Schaefer, J. Phys. Chem., 99, No. 8, 2324–2328 (1995).
M. T. Nguyen, Coord. Chem. Rev., 244, Nos. 1–2, 93–113 (2003).
L. Y. Bruney, T. M. Bledson, and D. L. Strout, Inorg. Chem., 42, No. 24, 8117–8120 (2003).
D. L. Strout, J. Phys. Chem. A, 108, No. 49, 10911–10916 (2004).
S. E. Sturdivant, F. A. Nelson, and D. L. Strout, J. Phys. Chem. A, 108, No. 34, 7087–7090 (2004).
M. Tobita and R. J. Bartlett, J. Phys. Chem. A, 105, No. 16, 4107–4113 (2001).
F. J. Owens, J. Mol. Struct. Theochem, 623, Nos. 1–3, 197–201 (2003).
L. Gagliardi and G. Orlandi, J. Chem. Phys., 114, No. 24, 10733 (2001).
T. K. Ha, O. Suleimenov, and M. T. Nguyen, Chem. Phys. Lett., 315, Nos. 5–6, 327–334 (1999).
D. L. Strout, J. Phys. Chem. A, 108, No. 13, 2555–2558 (2004).
L. J. Wang and M. Z. Zgierski, Chem. Phys. Lett., 376, Nos. 5–6, 698–703 (2003).
H. Zhou, N.-B. Wong, G. Zhou, and A. Tian, J. Phys. Chem. A, 110, No. 10, 3845–3852 (2006).
K. Grishakov, K. Katin, M. A. Gimaldinova, and M. Naslov, Lett. Mater., 9, No. 3, 366–369 (2019).
Q. Guo, B. He, and H. Zhou, J. Mol. Graph. Model, 96, 107508 (2020).
H. Zhou and N. B. Wong, Chem. Phys. Lett., 449, Nos. 4–6, 272–275 (2007).
V. B. Merinov, J. Struct. Chem., 62, No. 5, 661–670 (2021).
K. P. Katin, V. B. Merinov, A. I. Kochaev, et al., Computation, 8, No. 4, 91 (2020).
T. Yildirim, P. M. Gehring, D. A. Neumann, et al., Carbon, 36, Nos. 5–6, 809–815 (1998).
P. Liu, H. Cui, and G. W. Yang, Cryst. Growth Des., 8, No. 2, 581–586 (2008).
N. N. Degtyarenko, K. P. Katin, and M. M. Maslov, Phys. Solid State, 56, No. 7, 1467–1471 (2014).
K. P. Katin, M. B. Javan, A. I. Kochaev, et al., ChemistrySelect, 4, No. 33, 9659–9665 (2019).
K. P. Katin and M. M. Maslov, J. Phys. Chem. Solids, 108, 82–87 (2017).
M. A. Gimaldinova, M. M. Maslov, and K. P. Katin, CrystEngComm, 20, No. 30, 4336–4344 (2018).
A. D. Becke, J. Chem. Phys., 98, No. 7, 5648 (1993).
T. Lecklider, EE Eval. Eng., 50, No. 11, 36 (2011).
R.Krishnan, J. S. Binkley, R. Seeger, and J. A. Pople, J. Chem. Phys., 72, No. 1, 650 (1980).
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, et al., J. Comput. Chem., 14, No. 11, 1347–1363 (1993).
T. Yanai, D. P. Tew, and N. C. Handy, Chem. Phys. Lett., 393, Nos. 1–3, 51–57 (2004).
Chemcraft – graphical software for visualization of quantum chemistry computations [Electronic resource]; URL: https://www.chemcraftprog.com.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Izvestiya Vysshikh Uchebnykh Zavedenii, Fizika, No. 12, pp. 60–70, December, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Merinov, V.B. Structure and Optical Properties of High-Energy Nitrogen Clusters and Dimers on their Basis. Russ Phys J 65, 2109–2119 (2023). https://doi.org/10.1007/s11182-023-02879-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11182-023-02879-3