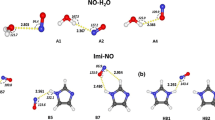
The mechanism of influence of molecular oxygen on the probability of ortho-para conversion of water molecules and its relation to water magnetization are considered within the framework of the concept of paramagnetic spin catalysis. Matrix elements of the hyperfine ortho-para interaction via the Fermi contact mechanism are calculated, as well as the Maliken spin densities on water protons in H2O and O2 collisional complexes. The mechanism of penetration of the electron spin density into the water molecule due to partial spin transfer from paramagnetic oxygen is considered. The probability of ortho-para conversion of the water molecules is estimated by the quantum chemistry methods. The results obtained show that effective ortho-para conversion of the water molecules is possible during the existence of water-oxygen dimers. An external magnetic field affects the ortho-para conversion rate given that the wave functions of nuclear spin sublevels of the water protons are mixed in the complex with oxygen.
Similar content being viewed by others
References
V. I. Klassen, Magnetization of Water Systems, Nauka, Moscow (1982).
A. Szkatula, M. Balanda, and M. Kopec, Eur. J. Appl. Phys., 18, 41–51 (2002).
L. Holysz, M. Chibowski, and E. Chibowski, Colloids Surf., A208, 231–240 (2002).
R. Gehr, Z. A. Zhai, J. A. Finch, et al., Water Res., 29, 933–940 (1995).
S. Knez and C. J. Pohar, Colloid Interface Sci., 281, 377–388 (2005).
R. E. Herzog, Q. Shi, J. N. Patil, et al., Langmuir, 5, 861–867 (1989).
Cz. E. Tomba, K. W. Busch, and M. A. Busch, Colloid Polym. Sci., 269, 278–289 (1991).
K. Higashitani, A. Kage, S. Katamura, et al., J. Colloid Interface Sci., 156, 90–95 (1993).
K. Higashitani, H. Iseri, K. Okuhara, et al., J. Colloid Interface Sci., 172, 383–388 (1995).
K. Higashitani and J. Oshitani, J. Colloid Interface Sci., 204, 363–368 (1998).
Ya. F. Bondarenko and E. Z. Gak, Electromagnetic Phenomena in Natural Water [in Russian], Gidrometeoizdat, Leningrad (1984).
G. F. Plekhanov, ed., Introduction to Electromagnetic Biology [in Russian], Publishing House of Tomsk State University, Tomsk (1979).
A. M. Demetskii and A. G. Alekseev, Artificial Magnetic Fields in Medicine [in Russian], Belarus, Minsk (1981).
I. Otsuka and S. Ozeki, J. Phys. Chem. B, 110, 1509–1512 (2006).
S. Ozeki and I. Otsuka, J. Phys. Chem. B, 110, 20067–20072 (2006).
S. M. Pershin, Nanostr. Mat. Fiz. Model., 7, 103–120 (2012).
A. F. Bunkin, A. A. Nurmatov, S. M. Pershin, et al., J. Ram. Spectrosc., 36, 145–147 (2005).
A. F. Bunkin, A. A. Nurmatov, and S. M. Pershin, Physics-Uspekhi, 49, 855–861 (2006).
K. Kuyanov-Prozument, M. Y. Choi, and A. F. Vilesov, J. Chem. Phys., 132, 014304 (7 pp.) (2010).
G. Buntkowsky, H.-H. Limbach, B. Walaszek, et al., Z. Phys. Chem., 222, 1049–1063 (2008).
X. Michout, A.-M. Vasserot, and L. Abouaf-Marguin, Vibr. Spectrosk., 34, 83–93 (2004).
P. L. Chapovsky and L. J. F. Hermans, Annu. Rev. Phys. Chem., 50, 315–345 (1999).
B. F. Minaev and H. Ågren, J. Phys. Chem., 99, 8936–8940 (1995).
B. Minaev, A. Baryshnikova, and W.-H. Sun, J. Organometal. Chem., 811, 48–65 (2016).
B. F. Minaev, Chem. Phys., 483–484, 84–95 (2017).
B. F. Minaev and H. Ågren, Int. J. Quant. Chem., 57, 519–532 (1996).
A. Sabu, S. Kondo, R. Saito, et al., J. Phys. Chem. A, 109, 1836–1842 (2005).
R. J. Wheatley and A. H. Harvey, J. Chem. Phys., 127, 074303 (8 pp.) (2007).
R. R. Valiev and B. F. Minaev, J. Mol. Model., 22, 214–222 (2016).
A. A. Granovsky, Firefly version 8.1.1, http://www.classic.chem.msu.su.
TURBOMOLE V7.0 2016, http://www.turbomole.com .
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al. Gaussian, Revision E.01, Gaussian Inc., Wallingford CT (2009).
J. J. P. Stewart, J. Comput. Chem., 10, 209–220 (1989).
P. W. Atkins and R. S. Friedman, Molecular Quantum Mechanics, Oxford University Press (2005).
B. F. Minaev, N. A. Murugan, and H. Ågren, Int. J. Quant. Chem., 13, 1847–1867 (2013).
Yu. A. Serebrennikov and B. F. Minaev, Chem. Phys., 114, 359–367 (1987).
V. V. Melnikov, E. V. Lavrov, Russ. Phys. J., 59, 2168–2170 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Izvestiya Vysshikh Uchebnykh Zavedenii, Fizika, No. 3, pp. 95–102, March, 2017.
Rights and permissions
About this article
Cite this article
Valiev, R.R., Minaev, B.F. Influence of Molecular Oxygen on Ortho-Para Conversion of Water Molecules. Russ Phys J 60, 485–493 (2017). https://doi.org/10.1007/s11182-017-1098-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11182-017-1098-3