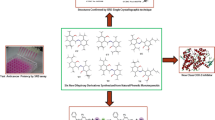
Abstract
A series of ligands were synthesized by crotonic condensation of nicotinaldehyde at the C(2) position of various tritepenoids and were then involved in the complex formation with PdII to prepare finally five new compounds. The cytotoxic activity evaluation in the A549 cell line (adenocarcinomic human alveolar basal epithelial cells) and the HeLa cell line (human cervical carcinoma cells) using the MTT assay demonstrated that methyl 3-oxo-2-(pyridin-3-ylmethylidene)-lup-20(29)-en-28-oate exhibits moderate cytotoxicity (IC50 is 17.34–20.02 µmol L−1).
Similar content being viewed by others
References
T. J. Carneiro, A. S. Martins, M. P. M. Marques, A. M. Gil, Front. Oncol., 2020, 10, 590970; DOI: https://doi.org/10.3389/fonc.2020.590970.
T. E. Kokina, V. Y. Komarov, L. A. Glinskaya, L. A. Sheludyakova, Y. A. Eremina, A. V. Tkachev, S. N. Bizyaev, L. S. Klyushova, Russ. J. Struct. Chem., 2020, 61, 44; DOI: https://doi.org/10.1134/S0022476620010059.
Th. Scattolin, V. A. Voloshkin, F. Visentin, S. P. Nolan, Cell Rep. Phys. Sci., 2021, 2, 100446; DOI: https://doi.org/10.1016/j.xcrp.2021.100446.
O. A. Zalevskaya, Y. A. Gur’eva, A. V. Kutchin, Inorg. Chim. Acta., 2021, 527, 120593; DOI: https://doi.org/10.1016/j.ica.2021.120593.
V. P. Boyarskii, A. S. Mikherdov, S. V. Baikov, P. Y. Savko, R. V. Suezov, R. E. Trifonov, Pharm. Chem. J., 2021, 55, 130; DOI: https://doi.org/10.1007/s11094-021-02393-1.
A. A. Batyrenko, O. V. Mikolaichuk, G. K. Ovsepyan, A. V. Protas, I. V. Kornyakov, E. V. Lider, Yu. A. Eremina, T. S. Khlebnikova, F. A. Lakhvich, R. E. Trifonov, Russ. J. Gen. Chem., 2021, 91, 666; DOI: https://doi.org/10.1134/S1070363221040149.
M. S. Denisov, O. N. Gagarskikh, Russ. J. Gen. Chem., 2021, 91, 1354; DOI: https://doi.org/10.1134/S1070363221070136.
T. V. Baulinaa, I. Yu. Kudryavtseva, O. V. Bykhovskayaa, M. P. Pasechnika, L. V. Anikinaa, A. V. Vologzhaninaa, V. A. Kochmarika, V. K. Brel, Russ. J. Gen. Chem., 2021, 91, 2052; DOI: https://doi.org/10.1134/S1070363221100194.
N. Van-Ha, Ph. T. T. Hien, D. T. Data, Do T. Thao, Mendeleev Commun., 2022, 32, 594; DOI: https://doi.org/10.1016/j.mencom.2022.09.008.
Ya. A. Gur’eva, O. A. Zalevskaya, N. S. Nikolaeva, Yu. R. Aleksandrova, E. Yu. Yandulova, M. E. Neganova, A. V. Kutchin Russ. Chem. Bull., 2023, 72, 793; DOI: 1066-5285/23/7203-0793.
O. Zalevskaya, Ya. Gur’eva, A. Kutchin, K. A. Hansford, Antibiotics, 2020, 9, 277; DOI: https://doi.org/10.3390/antibiotics9050277.
M. A. Nazarov, I. A. Tolmacheva, D. V. Eroshenko, O. A. Maiorova, M. V. Dmitriev, V. V. Grishko, Chem. Heterocycl. Compd., 2020, 56, 1321; DOI: https://doi.org/10.1007/s10593-020-02817-y.
M. N. Gorbunova, G. F. Krainova, A. O. Voronina, Khimiya rastitel’nogo syr’ya [Chemistry of Plant Raw Materials], 2020, 1, 49; DOI: https://doi.org/10.14258/jcprm.2020015609 (in Russian).
G. V. Giniyatullina, A. V. Petrova, A. G. Mustafin, Z. R. Zileeva, U. Sh. Kuzmina, Yu. V. Vakhitova, O. B. Kazakova, ChemistrySelect, 2021, 6, 13253; DOI: https://doi.org/10.1002/slct.202101687.
M. S. Denisov, V. A. Glushkov, Russ. Chem. Bull., 2020, 69, 2013; DOI: https://doi.org/10.1007/s11172-020-2993-2.
M. S. Denisov, M. V. Dmitriev, O. N. Gagarskikh, V. A. Glushkov, Chem. Nat. Compd., 2022, 58, 307; DOI: https://doi.org/10.1007/s10600-022-03665-2.
M. S. Denisov, Russ. J. Gen. Chem., 2023, 91, 37; DOI: https://doi.org/10.1134/S1070363223010061.
M. O. Tserfas, Yu. V. Kuznetsov, V. V. Knyazev, I. S. Levina, I. V. Zavarzin, Russ. Chem. Bull., 2022, 71, 1806; DOI: https://doi.org/10.1007/s11172-022-3593-0.
O. A. Koroleva, Yu. V. Dutikova, A. V. Trubnikov, F. A. Zenov, E. V. Manasova, A. A. Shtil, A. V. Kurkin, Russ. Chem. Bull., 2022, 71, 2310; DOI: https://doi.org/10.1007/s11172-022-3659-z.
V. V. Grishko, A. V. Nazarov, I. A. Tolmacheva, E. V. Tarasova, I. B. Ivshina, Chem. Nat. Compd., 2014, 50, 857; DOI: https://doi.org/10.1007/s10600-014-1100-z.
Zh. Liu, J. J. Swidorski, B. Nowicka-Sans, B. Terry, T. Protack, Z. Lin, H. Samanta, Sh. Zhang, Zh. Li, D. D. Parker, S. Rahematpura, S. Jenkins, B. R. Beno, M. Krystal, N. A. Meanwell, I. B. Dicker, A. Regueiro-Ren, Bioorg. Med. Chem., 2016, 24, 1757; DOI: https://doi.org/10.1016/j.bmc.2016.03.001.
Z. I. Galimova, M. S. Babaev, A. N. Lobov, O. B. Kazakova, Russ. J. Org. Chem., 2021, 57, 1012; DOI: https://doi.org/10.1134/S1070428021060208.
An Shen, Ch. Ni, Yu-C. Cao, H. Zhou, G.-H. Song, X.-F. Ye, Tetrahedron Lett., 2014, 55, 3278; DOI: https://doi.org/10.1016/j.tetlet.2014.04.044.
N. L. Bazyakina, V. M. Makarov, M. V. Moskalev, E. V. Baranov, A. S. Bogomyakov, V. I. Ovcharenko, I. L. Fedushkin, Mendeleev Commun., 2022, 32, 759; DOI: https://doi.org/10.1016/j.mencom.2022.11.017.
M. D. Hall, K. A. Telma, Ki-E. Chang, T. D. Lee, J. P. Madigan, J. R. Lloyd, I. S. Goldlust, J. D. Hoeschele, M. M. Gottesman, Cancer Res., 2014, 74, 3913; DOI: https://doi.org/10.1158/0008-5472.CAN-14-0247.
M. Neganova, A. Semakov, Yu. Aleksandrova, E. Yaduliva, S. Puhov, L. Anikina, S. Klochkov, Biomedicines, 2021, 9, 547; DOI: https://doi.org/10.3390/biomedicines9050547.
M. S. Denisov, Perm Federal Research Center Journal, 2021, 4, 6; DOI: https://doi.org/10.7242/2658-705X/2021.4.1.
M. R. Ibatullina, E. P. Zhil’tsova, N. V. Kulik, A. P. Lyubina, S. K. Amerhanova, A. D. Voloshina, S. S. Lukashenko, N. Kh. Safina, L. Ya. Zakharova, Russ. Chem. Bull., 2022, 71, 314; DOI: https://doi.org/10.1007/s11172-022-3413-6.
V. G. Vlasenko, A. S. Burlov, M. S. Milutka, Yu. V. Koshchienko, A. I. Uraev, V. A. Lazarenko, N. I. Makarova, A. V. Metelitsa, A. A. Zubenko, D. A. Garnovskii, Russ. J. Coord. Chem., 2023, 49, 148; DOI: https://doi.org/10.1134/S1070328422700221.
V. Korovin, A. V. Tkachev, Russ. Chem. Bull., 2001, 50, 304; DOI: https://doi.org/10.1023/A:1009598805986.
L.-W. Qian, J. Zhang, J.-H. Liu, B.-Ya. Yu, Tetrahedron Lett., 2009, 50, 2193; DOI: https://doi.org/10.1016/j.tetlet.2009.02.137.
H. Yamashita, M. Matsuzaki, Yu. Kurokawa, T. Nakane, M. Goto, K.-H. Lee, T. Shibata, H. Bandoa, K. Wad, Phytochem. Lett., 2019, 31, 140; DOI: https://doi.org/10.1016/j.phytol.2019.03.015.
O. Callies, L. M. Bedoya, M. Beltran, A. Muñ, P. O. Caldero, A. A. Osorio, I. A. Jimenez, J. Alcamí, I. L. Bazzocchi, J. Nat. Prod., 2015, 78, 1045; DOI: https://doi.org/10.1021/np501025r.
C. C. Laev, N. F. Salakhudinov, in Preparativnaya khimiya terpenoidov, Ch. 3. Triterpenoidy: lupeol, betulin, betulinovaya kislota, oleanolovaya kislota, moronovaya kislota, ursulovaya kislota, glitsiretovaya kislota, bosvellievaya kislota [Preparative Chemistry of Terpenoids. Part 3. Triterpenoids: Lupeol, Betulin, Betulinic Acid, Oleanolic Acid, Moronic Acid, Ursolic Acid, Glycyrrhetinic Acid, Boswellic Acid], Akademizdat, Novosibirsk, 2016, p. 99 (in Russian).
N. A. Dangroo, J. Singh, S. K. Ratha, N. Gupta, A. Qayum, Sh. Singh, P. L. Sangwan, Steroids, 2017, 123, 1; DOI: https://doi.org/10.1016/j.steroids.2017.04.002.
M. V. Kaverin, P. A. Morozova, L. V. Snegur, Russ. Chem. Bull., 2022, 71, 2236; DOI: https://doi.org/10.1007/s11172-022-3651-7.
CrysAlisPro, Rigaku Oxford Diffraction, 2022, Version 1.171.42.74a.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339; DOI: https://doi.org/10.1107/S0021889808042726.
G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv. 2015, A71, 3. DOI: https://doi.org/10.1107/S2053273314026370.
G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, C71, 3; DOI: https://doi.org/10.1107/S2053229614024218.
B. M. F. Gonçalves, J. A. R. Salvador, S. Marín, M. Cascante, Eur. J. Med. Chem., 2016. 114. 101; DOI: https://doi.org/10.1016/j.ejmech.2016.02.057.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no competing interests.
Additional information
The study was performed within the framework of the state assignment (state registration number 122012400109-8). The work was carried out using the equipment of the Core Facilities Center “Research of Materials and Matter” at the Perm Federal Research Center of the Ural Branch of the Russian Academy of Sciences (PFRC UB RAS).
We are grateful to O. A. Maiorova (PFRC UB RAS) for measuring NMR spectra, I. A. Borisova (PFRC UB RAS) for recording IR spectra, M. V. Dmitriev (Perm State University) for performing the X-ray diffraction study, and Yu. A. Beloglazova (PFRC UB RAS) for performing the MTT assay.
No human or animal subjects were used in this research.
Based on the materials of the XII All-Russian Scientific Conference “Chemistry and Technology of Plant Substances” with international participation and the School for Young Scientists (November 29–December 2, 2022, Kirov, Russia).
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, Vol. 72, No. 9, pp. 2206–2214, September, 2023.
Rights and permissions
About this article
Cite this article
Denisov, M.S., Eroshenko, D.V. Synthesis of 2-nicotinylidene triterpenoids: structure, complexation with palladium, and in vitro cytotoxic activity. Russ Chem Bull 72, 2206–2214 (2023). https://doi.org/10.1007/s11172-023-4017-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-023-4017-5