Abstract
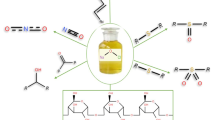
The oxidation of alkylphenols (2,6-dimethylphenol, 3,5-dimethylphenol, 2-iso-bornylphenol, 2-isobornyl-5-methylphenol, 2-isobornyl-6-methylphenol) with chlorine dioxide in water and dichloromethane was studied. The specific features of alkylphenol oxidation under different reaction conditions were revealed. The reaction of alkylated phenols with chlorine dioxide resulted in the formation of quinones and products of oxidative chlorination. The yields of quinones were found to depend on the position of substituents in the aromatic ring of the starting phenols.
Similar content being viewed by others
References
Election of the Full Members of the Russian Academy of Sciences, Russ. Chem. Bull., 2022, 71, 1559; DOI: https://doi.org/10.1007/s11172-022-3565-4.
L. L. Frolova, A. V. Popov, S. A. Rubtsova, A. V. Kutchin, Chem. Nat. Compd., 2008, 44, 724; DOI: https://doi.org/10.1007/s10600-009-9190-8.
L. L. Frolova, A. V. Popov, L. V. Bezuglaya, I. N. Alekseev, P. A. Slepukhin, A. V. Kutchin, Russ. J. Gen. Chem., 2014, 84, 853; DOI: https://doi.org/10.1134/s1070363214050120.
I. V. Loginova, S. A Rubtsova, A. V. Kuchin, Chem. Nat. Compd., 2008, 44, 752; DOI: https://doi.org/10.1007/s10600-009-9182-8.
O. M. Lezina, S. A. Rubtsova, A. V. Kuchin, Russ. Chem. Bull., 2003, 52, 1877; DOI: https://doi.org/10.1023/A:1026093713037.
O. M. Lezina, S. A. Rubtsova, A. V. Kuchin, Russ. J. Org. Chem., 2011, 47, 1249.
O. M. Lezina, O. N. Grebenkina, D. V. Sudarikov, Yu. V. Krymskaya, S. A. Rubtsova, A. V. Kutchin, Russ. J. Org. Chem., 2015, 51, 1359; DOI: https://doi.org/10.1134/S1070428015100012.
E. S. Izmestev, O. N. Grebenkina, S. A. Patov, S. A. Rubtsova, A. V. Kutchin, Russ. Chem. Bull., 2014, 63, 2067; DOI: https://doi.org/10.1007/s11172-014-0702-8.
A. V. Kutchin, S. A. Rubtsova, I. V. Loginova, Russ. Chem. Bull., 2001, 50, 432; DOI: https://doi.org/10.1023/A:1011348804933.
A. V. Kutchin, S. A. Rubtsova, L. P. Karmanova, S. N. Subbotina, I. V. Loginova, Russ. Chem. Bull., 1998, 47, 2051; DOI: https://doi.org/10.1007/BF02494537.
I. V. Loginova, E. V. Ashikhmina, S. A. Rubtsova, Yu. V. Krymskaya, A. V. Kuchin, Russ. J. Org. Chem., 2008, 44, 1773; DOI: https://doi.org/10.1134/S1070428008120087.
I. V. Loginova, K. S. Rodygin, S. A. Rubtsova, A. V. Kuchin, P. A. Slepukhin, V. A. Polukeev, Russ. J. Org. Chem., 2011, 47, 124; DOI: https://doi.org/10.1134/S107042801302005X.
Yu. V. Krymskaya, I. V. Loginova, S. A. Rubtsova, A. V. Kuchin, Russ. J. Org. Chem., 2013, 49, 204; DOI: https://doi.org/10.1134/S1070428011010167.
I. V. Loginova, I. Y. Chukicheva, A. V. Kuchin, Rus. J. Org. Chem., 2011, 47, 1501; DOI: https://doi.org/10.1134/S1070428011100083.
I. M. Ganiev, E. S. Suvorkina, N. N. Kabal’nova, Russ. Chem. Bull., 2003, 52, 1123; DOI: https://doi.org/10.1023/A:1024905223801.
I. M. Ganiev, E. S. Suvorkina, N. N. Kabal’nova, Russ. Chem. Bull., 2004, 53, 2281; DOI: https://doi.org/10.1007/s11172-005-0114-x.
J. E. Wajon, D. H. Rosenblatt, E. P. Burrows, Environ. Sci. Technol., 1982, 16, 396; DOI: https://doi.org/10.1021/es00101a006.
M. Kawamukai, Biosci. Biotechnol. Biochem., 2018, 82, 963; DOI: https://doi.org/10.1080/09168451.2018.1433020.
V. V. Plemenkov, Vvedeniye v khimiyu prirodnykh soedineniy [Introduction to the Chemistry of Natural Compounds], Kazan, 2001, 376 pp. (in Russian).
N. G. Krylova, T. A. Kulahava, G. N. Semenkova, O. I. Shadyro, S. N. Cherenkevich, Proc. Nat. Acad. Sci. Belarus, Biol. Ser., 2014, 1015; DOI: http://elib.bsu.by/handle/123456789/216950.
I. Abraham, R. Joshi, P. Pardasani, R. T. Pardasani, J. Braz. Chem. Soc., 2011, 22, 385; DOI: https://doi.org/10.1590/s0103-50532011000300002.
I. V. Smolyaninov, V. V. Kuzmin, M. V. Arsenyev, S. A. Smolyaninova, A. I. Poddel’sky, N. T. Berberova, Russ. Chem. Bull., 2017, 66, 1217; DOI: https://doi.org/10.1007/s11172-017-1876-7.
M. J. Abad Martínez, P. Bermejo Benito, Stud. Nat. Prod. Chem., 2005, 30, 303, 366; DOI: https://doi.org/10.1016/S1572-5995(05)80036-5.
J. L. Bolton, T. Dunlap, Chem. Res. Toxicol., 2017, 30, 13; DOI: https://doi.org/10.1021/acs.chemrestox.6b00256.
E. N. da Silva, Jr., G. A. M. Jardim, C. Jacob, U. Dhawa, L. Ackermann, S. L. de Castro, Eur. J. Med. Chem., 2019, 179, 863; DOI: https://doi.org/10.1016/j.ejmech.2019.06.056.
V. F. Ferreira, A. S. de Carvalho, P. G. Ferreira, C. G. S. Lima, F. de C da Silva, Eur. J. Med. Chem., 2021, 17, 1073; DOI: https://doi.org/10.2174/1573406416666201106104756.
B. S. Bhatkhande, M. V. Adhikari, S. D. Samant, Ultrason. Sonochem., 2002, 9, 31; DOI: https://doi.org/10.1016/S1350-4177(01)00097-9.
G. Haeseler, S. Gudehus, J. Bufler, R. Dengler, M. Leuwer, Eur. J. Anaesthesiol., 2006, 23, 190; DOI: https://doi.org/10.1017/S0265021505002176.
H. Awano, S. Sakai, T. Kuriyama, Y. Ohba, Synth. Met., 1995, 70, 1121; DOI: https://doi.org/10.1016/0379-6779(94)02782-T.
C. Nallaiah, J. A. Strickson, Tetrahedron, 1986, 42, 4083; DOI: https://doi.org/10.1016/S0040-4020(01)87565-7.
L. I. Smith, W. B. Irwin, C. O. Guss, J. Am. Chem. Soc., 1941, 63, 1036; DOI: https://doi.org/10.1021/ja01849a042.
R. Lesser, Ber. Dtsch. Chem. Ges. B, 1923, 56, 963; DOI: https://doi.org/10.1002/cber.1923056427.
A. Podgorsek, M. Jurisch, S. Stavber, M. Zupan, J. Iskra, J. A. Gladysz, J. Org. Chem., 2009, 74, 3133; DOI: https://doi.org/10.1021/jo900233h.
P. Weiss, M. G. Cordasco, W. Carman, L. Reiner, J. Am. Pharm. Assoc. (1912–1977), 1951, 40, 267; DOI: https://doi.org/10.1002/jps.3030400605.
Preparativnaya organicheskaya khimiya [Preparative organic chemistry], Khimicheskaya literatura, Moscow, 1959, 888 pp. (in Russian).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (Russian state assignment No. 122040600073-3) and the World-class Scientific and Educational Center “Russian Arctic: New Materials, Technologies and Research Methods”.
No human or animal subjects were used in this research.
The authors declare no competing interests.
Kuchin Alexander Vasilyevich, born in 1949, Doctor of Chemical Sciences, Professor, Chief Researcher of the Federal Research Centre “Komi Science Centre of the Ural Branch of the Russian Academy of Sciences” (Syktyvkar), was elected a corresponding member of the Russian Academy of Sciences in 2000, elected a full member of the Russian Academy of Sciences in 2022 (more information is given in the article1).
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, Vol. 72, No. 1, pp. 202–212, January, 2023.
Rights and permissions
About this article
Cite this article
Kutchin, A.V., Fedorova, I.V., Loginova, I.V. et al. Features of the use of ClO2 in the oxidation of some alkylphenols. Russ Chem Bull 72, 202–212 (2023). https://doi.org/10.1007/s11172-023-3725-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-023-3725-1