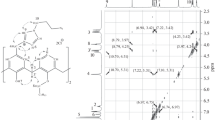
Abstract
Novel di- and tetra-substituted calix[4]arene derivatives bearing diacetylene moieties at the lower rim of macrocycle were synthesized via the Mitsunobu and Williamson reactions. The dealkylation of diacetylene moieties of calix[4]arene was for the first time discovered in the DMSO—hydrazine hydrate system. An important role in the dealkylation of diacetylene moieties is played by neighboring unsubstituted OH groups at the macrocycle. Blocking of OH groups with butyl substituents and elongation of the linker between the butadiynyl fragments and the macrocyclic platform significantly reduce the affinity of macrocycle to reductive cleavage. The unique reactivity of calix[4]arene in its reaction with hydrazine hydrate was demonstrated in comparison with a model conjugate of diacetylene with 4-tert-butylphenol. The reductive cleavage may proceed via the formation of pyrazole derivative of calix[4]arene. Pyrazole derivatives were the most reactive ones in the reaction with hydrazine hydrate among the studied isostructural calixarenes containing an active α-arylmethyleneoxy moiety. Results of performed quantum chemical calculations allowed us to propose a scheme for the reductive cleavage of oxymethylene derivatives of pyrazole with hydrazine hydrate.
Similar content being viewed by others
References
W. Shi, A. Lei, Tetrahedron Lett., 2014, 55, 2763; DOI: https://doi.org/10.1016/j.tetlet.2014.03.022.
X. Quian, B. Stadler, Chem. Mater., 2019, 31, 1196; DOI: https://doi.org/10.1021/acs.chemmater.8b05185.
H. Jiang, W. Zeng, Y. Li, W. Wu, L. Huang, W. Fu, J. Org. Chem., 2012, 77, 5179; DOI: https://doi.org/10.1021/jo300692d.
L. Wang, X. Yu, X. Feng, M. Bao, J. Org. Chem., 2013, 78, 1693; DOI: https://doi.org/10.1021/jo302732v.
Q. Zheng, R. Hua, Tetrahedron Lett., 2010, 51, 4512; DOI: https://doi.org/10.1016/j.tetlet.2010.06.092.
K. T. Potts, S. A. Nye, K. A. Smith, J. Org. Chem., 1992, 57, 3895; DOI: https://doi.org/10.1021/jo00040a032.
Calixarenes and Beyond, Eds P. Neri, J. L. Sessler, M.-X. Wang, Springer, Cham, 2016, 1062 pp.; DOI: https://doi.org/10.1007/978-3-319-31867-7_33.
S. E. Solovieva, V. A. Burilov, I. S. Antipin, Macroheterocycles, 2017, 10, 134; DOI: https://doi.org/10.6060/mhc170512a.
F. Nasuhi Pur, Mol. Diversity, 2016, 20, 781; DOI: https://doi.org/10.1007/s11030-016-9667-x.
J. Yang, J. Liu, Y. Wang, J. Wang, J. Incl. Phenom. Macrocycl. Chem., 2018, 90, 15; DOI: https://doi.org/10.1007/s10847-017-0766-9.
V. A. Burilov, I. M. Bogdanov, R. I. Garipova, A. A. Volodina, D. A. Mironova, V. G. Evtugyn, S. E. Solovieva, I. S. Antipin, Russ. Chem. Bull., 2022, 71, 131; DOI: https://doi.org/10.1007/s11172-022-3386-5.
A. A. Muravev, A. S. Agarkov, F. B. Galieva, A. T. Yakupov, O. B. Bazanova, I. Kh. Rizvanov, A. V. Shokurov, A. V. Zaitseva, S. L. Selektor, S. E. Solovieva, I. S. Antipin, Russ. Chem. Bull., 2020, 69, 339; DOI: https://doi.org/10.1007/s11172-020-2766-y.
V. A. Burilov, D. A. Mironova, R. R. Ibragimova, V. G. Evtugyn, Yu. N. Osin, S. E. Solovieva, I. S. Antipin, BioNanoSci., 2018, 8, 337; DOI: https://doi.org/10.1007/s12668-017-0484-1.
N. J. Maher, H. Diao, J. O’Sullivan, E. Fadda, F. Heaney, J. McGinley, Tetrahedron, 2015, 71, 9223; DOI: https://doi.org/10.1016/j.tet.2015.10.045.
A. Gorbunov, D. Cheshkov, V. Kovalev, I. Vatsouro, Chem. Eur. J., 2015, 21, 9528; DOI: https://doi.org/10.1002/chem.201500946.
A. K. Agrahari, A. K. Singh, A. S. Singh, M. Singh, P. Maji, S. Yadav, S. Rajkhowa, P. Prakash, V. K. Tiwari, New J. Chem., 2020, 44, 19300; DOI:https://doi.org/10.1039/d0nj02591g.
A. A. Muravev, S. E. Solovieva, F. B. Galieva, O. B. Bazanova, I. K. Rizvanov, K. A. Ivshin, O. N. Kataeva, S. E. Matthewsc, I. S. Antipin, RSC Adv., 2018, 8, 32765; DOI: https://doi.org/10.1039/c8ra06349d.
V. Burilov, A. Valiyakhmetova, D. Mironova, R. Safiullin, M. Kadirov, K. Ivshin, O. Kataeva, S. Solovieva, I. Antipin, RSC Adv., 2016, 6, 44873; DOI: https://doi.org/10.1039/C6RA07555J.
Y. Kashiwame, T. Ikariya, S. Kuwata, Polyhedron, 2021, 197, 115036; DOI: https://doi.org/10.1016/j.poly.2021.115036.
V. A. Burilov, G. A. Fatikhova, M. N. Dokuchaeva, R. I. Nugmanov, D. A. Mironova, P. V. Dorovatovskii, V. N. Khrustalev, S. E. Solovieva, I. S. Antipin, Beilstein J. Org. Chem., 2018, 14, 1980; DOI: https://doi.org/10.3762/bjoc.14.173.
K. Tsutsumi, S. Ogoshi, S. Nishiguchi, H. Kurosawa, J. Am. Chem. Soc., 1998, 120, 1938; DOI: https://doi.org/10.1021/om700909k.
H. Li, L. Wang, M. Yang, Y. Qi, Catal. Commun., 2012, 17, 179; DOI: https://doi.org/10.1016/j.catcom.2011.10.027.
L. S. Arora, H. M. Chawla, M. Shahid, N. Pant, Org. Prep. Proced. Int., 2017, 49, 228; DOI: https://doi.org/10.1080/00304948.2017.1320903.
V. A. Burilov, R. I. Nugmanov, R. R. Ibragimova, S. E. Solovieva, I. S. Antipin, A. I. Konovalov, Mendeleev Commun., 2013, 23, 113; DOI: https://doi.org/10.1016/j.mencom.2013.03.022.
E. A. Alekseeva, A. P. Luk’yanenko, A. I. Gren, Mendeleev Commun., 2012, 22, 263; DOI: https://doi.org/10.1016/j.mencom.2012.09.012.
D. N. Laikov, Chem. Phys. Lett., 1997, 281, 151; DOI: https://doi.org/10.1016/S0009-2614(97)01206-2.
D. N. Laikov, Chem. Phys. Lett., 2005, 416, 116; DOI: https://doi.org/10.1016/j.cplett.2005.09.046.
W. L. F. Armarego, C. L. L. Chai, Purification of Laboratory Chemicals, 6th ed., Elsevier, Oxford, 2009, 760 pp.; DOI: https://doi.org/10.1016/C2009-0-26589-5.
J. Schmidt-Leithoff, R. Brückner, Synlett, 2006, 16, 2641; DOI: https://doi.org/10.1055/s-2006-951473.
N. Iki, C. Kabuto, T. Fukushima, H. Kumagai, H. Takeya, S. Miyanari, T. Miyashi, S. Miyano, Tetrahedron, 2000, 56, 1437; DOI: https://doi.org/10.1016/S0040-4020(00)00030-2.
Funding
This work was financially supported by the Russian Science Foundation (Grant No. 21-73-10062).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dedicated to Academician of the Russian Academy of Sciences V. I. Ovcharenko on the occasion of his 70th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 7, pp. 1497–1505, July, 2022.
No human or animal subjects were used in this research.
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Burilov, V.A., Belov, R.N., Nugmanov, R.I. et al. Hydrazine-mediated C-O bond reductive cleavage in some bis- and mono-O-substituted derivatives of 4-tert-butylcalix[4]arene. Russ Chem Bull 71, 1497–1505 (2022). https://doi.org/10.1007/s11172-022-3556-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3556-5