Abstract
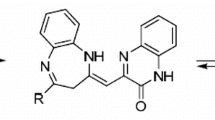
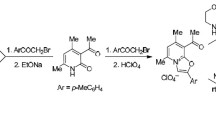
The mechanism of formation of methyl (4RS,6SR)-2-(4-bromophenyl)-5,5-dicyano-4,6-bis(4-methylphenyl)-1,4,5,6-tetrahydropyridine-3-carboxylate was estimated. Formation of 1,4,5,6-tetrahydropyridine moiety proceeds through a sequence of the Michael addition, the Mannich reaction, stereoselective cyclization to polysubstituted (2SR,3RS,4SR,6RS)-2-hydroxypiperidine, dehydration to (3RS,4SR,6RS)-3,4,5,6-tetrahydropyridine, and isomerization. Polysubstituted (4RS,6SR)-1,4,5,6-tetrahydropyridine was found to be thermodynamically more stable than isomeric (3RS,4SR,6RS)-3,4,5,6-tetrahydropyridine.
Similar content being viewed by others
References
R. Vardanyan, Piperidine-Based Drug Discovery, Ch. 1:1–82, Elsevier Ltd, Amsterdam, 2017.
N. M. Przhevalskii, R. K. Laypanov, G. P. Tokmakov, I. V. Lukina, D. A. Vershinkin, V. A. Tafeenko, Russ. Chem. Bull., 2021, 70, 555; DOI: https://doi.org/10.1007/s11172-021-3124-4.
I. A. Novakov, D. S. Sheikin, M. B. Navrotskii, A. S. Mkrtchyan, L. L. Brunilina, K. V. Balakin, Russ. Chem. Bull., 2020, 69, 1625; DOI: https://doi.org/10.1007/s11172-020-2946-9.
S. Goldmann, J. Stoltefuss, Angew. Chem., Int. Ed., 1991, 30, 1559; DOI: https://doi.org/10.1002/anie.199115591.
B. J. Epstein, K. Vogel, B. F. Palmer, Drugs, 2007, 67, 1309; DOI: https://doi.org/10.2165/00003495-200767090-00005.
M. Misra, S. K. Pandey, V. P. Pandey, J. Pandey, R. Tripathi, R. P. Tripathi, Bioorg. Med. Chem., 2009, 17, 625; DOI: https://doi.org/10.1016/j.bmc.2008.11.062.
I. Borza, G. Domany, Curr. Top. Med. Chem., 2006, 6, 687; DOI: https://doi.org/10.2174/156802606776894456.
R. Jain, D. Chen, R. J. White, D. V. Patel, Z. Yuan, Curr. Med. Chem., 2005, 12, 1607; DOI: https://doi.org/10.2174/0929867054367194.
L. Bazargan, S. Fouladdel, A. Shafiee, M. Amini, S. M. Ghaffari, E. Azizi, Cell Biol. Toxicol., 2008, 24, 165; DOI: https://doi.org/10.1007/s10565-007-9026-x.
S. R. M. D. Morshed, K. Hashimoto, Y. Murotani, M. Kawase, A. Shah, K. Satoh, H. Kikuchi, H. Nishikawa, J. Maki, H. Sakagami, Anticancer Res., 2005, 25, 2033.
A. Hilgeroth, H. Lilie, Eur. J. Med. Chem., 2003, 38, 495; DOI: https://doi.org/10.1016/S0223-5234(03)00060-6.
D. Schade, M. Lanier, E. Willems, K. Okolotowicz, P. Bushway, C. Wahlquist, C. Gilley, M. Mercola, J. R. Cashman, J. Med. Chem., 2012, 55, 9946; DOI: https://doi.org/10.1021/jm301144g.
B. Han, J.-L. Li, C. Ma, S.-J. Zhang, Y.-C. Chen, Angew. Chem., Int. Ed., 2008, 47, 9971; DOI: https://doi.org/10.1002/anie.200804183.
M. Rueping, A. P. Antonchick, Angew. Chem., Int. Ed., 2008, 47, 5836; DOI: https://doi.org/10.1002/anie.200801435.
J. Zhang, W.-J. Yang, J. Sun, C.-G. Yan, Eur. J. Org. Chem., 2015, 7571; DOI: https://doi.org/10.1002/ejoc.201501052.
R.-G. Han, Y. Wang, Y.-Y. Li, P.-F. Xu, Adv. Synth. Catal., 2008, 350, 1474; DOI: https://doi.org/10.1002/ejoc.201501052.
A. N. Vereshchagin, Russ. Chem. Bull., 2017, 66, 1765; DOI: https://doi.org/10.1007/s11172-017-1950-1.
H.-J. Wang, L.-P. Mo, Z.-H. Zhang, ACS Comb. Sci., 2011, 13, 181; DOI: https://doi.org/10.1021/co100055x.
N. R. Agrawal, S. P. Bahekar, P. B. Sarode, S. S. Zade, H. S. Chandak, RSC Adv., 2015, 5, 47053; DOI: https://doi.org/10.1039/C5RA08022C.
M. M. F. Ismail, A. M. Farrag, D. Abou-El-Ela, J. Heterocycl. Compd., 2020, 57, 3442; DOI: https://doi.org/10.1002/jhet.4064.
I. V. Dyachenko, V. D. Dyachenko, P. V. Dorovatovskii, V. N. Khrustalev, V. G. Nenajdenko, Russ. Chem. Bull., 2021, 70, 2145; DOI: https://doi.org/10.1007/s11172-021-3326-9.
M. N. Elinson, S. K. Feducovich, T. A. Zaimovskaya, A. N. Vereshchagin, S. V. Gorbunov, G. I. Nikishin, Russ. Chem. Bull., 2005, 54, 1593; DOI: https://doi.org/10.1007/s11172-006-0008-6.
A. N. Vereshchagin, M. N. Elinson, N. O. Stepanov, G. I. Nikishin, Mendeleev Commun., 2009, 19, 324; DOI: https://doi.org/10.1016/j.mencom.2009.11.010.
M. N. Elinson, A. N. Vereshchagin, N. O. Stepanov, A. I. Ilovaisky, A. Y. Vorontsov, G. I. Nikishin, Tetrahedron, 2009, 65, 6057; DOI: https://doi.org/10.1016/j.tet.2009.05.062.
M. N. Elinson, S. K. Feducovich, T. A. Zaimovskaya, A. N. Vereshchagin, G. I. Nikishin, Russ. Chem. Bull., 2003, 52, 2241; DOI: https://doi.org/10.1023/B:RUCB.0000011885.10104.da.
M. N. Elinson, A. N. Vereshchagin, F. V. Ryzhkov, Curr. Org. Chem., 2017, 21, 1427; DOI: https://doi.org/10.2174/1385272820666161017170200.
A. N. Vereshchagin, K. A. Karpenko, M. N. Elinson, E. O. Dorofeeva, A. S. Goloveshkin, M. P. Egorov, Mendeleev Commun., 2018, 28, 384; DOI: https://doi.org/10.1016/j.mencom.2018.07.014.
A. N. Vereshchagin, K. A. Karpenko, M. N. Elinson, A. S. Goloveshkin, I. E. Ushakov, M. P. Egorov, Res. Chem. Intermed., 2018, 44, 5623; DOI: https://doi.org/10.1007/s11164-018-3444-7.
A. N. Vereshchagin, K. A. Karpenko, M. N. Elinson, S. V. Gorbunov, A. M. Gordeeva, P. I. Proshin, A. S. Goloveshkin, M. P. Egorov, Monatsh. Chem., 2018, 149, 1979; DOI: https://doi.org/10.1007/s00706-018-2187-x.
A. N. Vereshchagin, K. A. Karpenko, M. N. Elinson, A. S. Goloveshkin, E. O. Dorofeeva, M. P. Egorov, Res. Chem. Intermed., 2020, 46, 1183; DOI: https://doi.org/10.1007/s11164-019-04027-4.
A. N. Vereshchagin, K. A. Karpenko, T. M. Iliyasov, M. N. Elinson, E. O. Dorofeeva, A. N. Fakhrutdinov, M. P. Egorov, Russ. Chem. Bull., 2018, 67, 2049; DOI: https://doi.org/10.1007/s11172-018-2327-9.
A. N. Vereshchagin, T. M. Iliyasov, K. A. Karpenko, V. A. Smirnov, I. E. Ushakov, M. N. Elinson, Chem. Heterocycl. Compd., 2021, 57, 929; DOI: https://doi.org/10.1007/s10593-021-03002-5.
M. Chakrabarty, S. Karmakar, S. Arima, Y. Harigaya, Heterocycles, 2007, 73, 795; DOI: https://doi.org/10.3987/COM-07-S(U)59.
A. A. Granovsky, Firefly version 8., 1997; http://classic.chem.msu.su/gran/firefly/index.html.
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. Su, T. L. Windus, M. Dupuis, J. A. MontgomeryJr, J. Comput. Chem., 1993, 14, 1347; DOI: https://doi.org/10.1002/jcc.540141112.
D. Rappoport, F. Furche, J. Chem. Phys., 2010, 133, 134105; DOI: https://doi.org/10.1063/1.3484283.
A. N. Vereshchagin, M. N. Elinson, M. P. Egorov, RSC Adv., 2015, 5, 98522; DOI: https://doi.org/10.1039/C5RA19690F.
Funding
The study was financially supported by the Russian Science Foundation (Project No. 17-73-20260).
Author information
Authors and Affiliations
Corresponding author
Additional information
No human or animal subjects were used in this research.
The authors declare no competing interests.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 1279–1283, June, 2022.
Rights and permissions
About this article
Cite this article
Karpenko, K.A., Iliyasov, T.M., Fakhrutdinov, A.N. et al. Study on formation mechanism of (4RS,6SR)-4,6-diaryl-5,5-dicyano-2-methyl-1,4,5,6-tetrahydropyridine-3-carboxylic esters. Russ Chem Bull 71, 1278–1283 (2022). https://doi.org/10.1007/s11172-022-3531-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3531-1