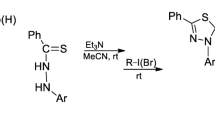
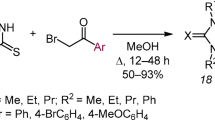
Abstract
The reaction of 1,4-disubstituted thiosemicarbazides with N,N-dimethylformamide dimethyl acetal afforded 1,4-disubstituted 1,2,4-triazolium-3-thiolates. Heating of the thiosemicarbazides with trimethyl orthoformate in the presence of Me3SiCl yielded 5-RNH-substituted 1,3,4-thiadiazolium salts and, in some cases, 1,4-disubstituted 3-sulfanyl-1,2,4-triazolium salts as by-products. It was found that the obtained heterocycles are capable of reversible interconversions depending on the medium acidity and the structure of substituent R. Aminothiadiazolium salts in the presence of bases underwent rearrangement into triazolium thiolates, except for the case when R = 2,6-diisopropylphenyl. Triazolium thiolates and sulfanyltriazolium salts were converted into aminothiadiazolium salts upon heating in an acidic medium. S-Alkylation of triazolium thiolates gave stable 3-alkylsulfanyl-1,2,4-triazolium salts, which can be used as NHC-proligands. Direct palladation of alkylsulfanyl triazolium salts was used to obtain the PEPPSI type Pd/NHC complexes.
Similar content being viewed by others
References
W. A. Herrmann, Angew. Chem., Int. Ed., 2002, 41, 1290; DOI: https://doi.org/10.1002/1521-3773(20020415)41:8<1290::AID-ANIE1290>3.0.CO;2-Y.
M. N. Hopkinson, C. Richter, M. Schedler, F. Glorius, Nature, 2014, 510, 485; DOI: https://doi.org/10.1038/nature13384.
P. Bellotti, M. Koy, M. N. Hopkinson, F. Glorius, Nat. Rev. Chem., 2021, 5, 711; DOI: https://doi.org/10.1038/s41570-021-00321-1.
G. C. Fortman, S. P. Nolan, Chem. Soc. Rev., 2011, 40, 5151; DOI: https://doi.org/10.1039/C1CS15088J.
R. D. J. Froese, C. Lombardi, M. Pompeo, R. P. Rucker, M. G. Organ, Acc. Chem. Res., 2017, 50, 2244; DOI: https://doi.org/10.1021/acs.accounts.7b00249.
C. Diner, M. G. Organ, Organometallics, 2019, 38, 66; DOI: https://doi.org/10.1021/acs.organomet.8b00818.
F. Nahra, D. J. Nelson, S. P. Nolan, Trends Chem., 2020, 2, 1096; DOI: https://doi.org/10.1016/j.trechm.2020.10.003.
T. Scattolin, S. P. Nolan, Trends Chem., 2020, 2, 721; DOI: https://doi.org/10.1016/j.trechm.2020.06.001.
Q. Zhao, G. Meng, S. P. Nolan, M. Szostak, Chem. Rev., 2020, 120, 1981; DOI: https://doi.org/10.1021/acs.chemrev.9b00634.
A. V. Astakhov, S. B. Soliev, V. M. Chernyshev, Russ. Chem. Bull., 2020, 69, 2073; DOI: https://doi.org/10.1007/s11172-020-3002-5.
S. B. Soliev, A. V. Astakhov, D. V. Pasyukov, V. M. Chernyshev, Russ. Chem. Bull., 2020, 69, 683; DOI: https://doi.org/10.1007/s11172-020-2818-3.
M. S. Denisov, M. V. Dmitriev, A. A. Gorbunov, V. A. Glushkov, Russ. Chem. Bull., 2019, 68, 2039; DOI: https://doi.org/10.1007/s11172-019-2664-3.
V. A. Glushkov, D. N. Babentzev, M. V. Dmitriev, I. A. Borisova, M. S. Denisov, Russ. Chem. Bull., 2021, 70, 122; DOI: https://doi.org/10.1007/s11172-021-3065-y.
R. Visbal, M. C. Gimeno, Chem. Soc. Rev., 2014, 43, 3551; DOI: https://doi.org/10.1039/C3CS60466G.
M. Elie, J. L. Renaud, S. Gaillard, Polyhedron, 2018, 140, 158; DOI: https://doi.org/10.1016/j.poly.2017.11.045.
C. A. Smith, M. R. Narouz, P. A. Lummis, I. Singh, A. Nazemi, C.-H. Li, C. M. Crudden, Chem. Rev., 2019, 119, 4986; DOI: https://doi.org/10.1021/acs.chemrev.8b00514.
W. Liu, R. Gust, Coord. Chem. Rev., 2016, 329, 191; DOI: https://doi.org/10.1016/j.ccr.2016.09.004.
M. Porchia, M. Pellei, M. Marinelli, F. Tisato, F. Del Bello, C. Santini, Eur. J. Med. Chem., 2018, 146, 709; DOI: https://doi.org/10.1016/j.ejmech.2018.01.065.
T. Zou, C.-N. Lok, P.-K. Wan, Z.-F. Zhang, S.-K. Fung, C.-M. Che, Curr. Opin. Chem. Biol., 2018, 43, 30; DOI: https://doi.org/10.1016/j.cbpa.2017.10.014.
T. Scattolin, V. A. Voloshkin, F. Visentin, S. P. Nolan, Cell Rep. Phys. Sci., 2021, 2, 100446; DOI: https://doi.org/10.1016/j.xcrp.2021.100446.
X. Liang, S. Luan, Z. Yin, M. He, C. He, L. Yin, Y. Zou, Z. Yuan, L. Li, X. Song, C. Lv, W. Zhang, Eur. J. Med. Chem., 2018, 157, 62; DOI: https://doi.org/10.1016/j.ejmech.2018.07.057.
M. Mora, M. C. Gimeno, R. Visbal, Chem. Soc. Rev., 2019, 48, 447; DOI: https://doi.org/10.1039/C8CS00570B.
D. M. Flanigan, F. Romanov-Michailidis, N. A. White, T. Rovis, Chem. Rev., 2015, 115, 9307; DOI: https://doi.org/10.1021/acs.chemrev.5b00060.
D. Janssen-Müller, C. Schlepphorst, F. Glorius, Chem. Soc. Rev., 2017, 46, 4845; DOI: https://doi.org/10.1039/C7CS00200A.
J. Wang, C. Zhao, J. Wang, ACS Catal., 2021, 11, 12520; DOI: https://doi.org/10.1021/acscatal.1c03459.
J. Liu, X.-N. Xing, J.-H. Huang, L.-Q. Lu, W.-J. Xiao, Chem. Sci., 2020, 11, 10605; DOI: https://doi.org/10.1039/D0SC03595E.
L. Marzo, Eur. J. Org. Chem., 2021, 2021, 4603; DOI: https://doi.org/10.1002/ejoc.202100261.
B. Wang, L. Qin, T. Mu, Z. Xue, G. Gao, Chem. Rev., 2017, 117, 7113; DOI: https://doi.org/10.1021/acs.chemrev.6b00594.
T. Welton, Biophys. Rev., 2018, 10, 691; DOI: https://doi.org/10.1007/s12551-018-0419-2.
N. Nasirpour, M. Mohammadpourfard, S. Zeinali Heris, Chem. Eng. Res. Des., 2020, 160, 264; DOI: https://doi.org/10.1016/j.cherd.2020.06.006.
M. A. Ortuño, N. López, Catal. Sci. Technol., 2019, 9, 5173; DOI: https://doi.org/10.1039/C9CY01351B.
C. Cerezo-Navarrete, P. Lara, L. M. Martínez-Prieto, Catalyst, 2020, 10, 1144; DOI: https://doi.org/10.3390/catal10101144.
V. M. Chernyshev, O. V. Khazipov, D. B. Eremin, E. A. Denisova, V. P. Ananikov, Coord. Chem. Rev., 2021, 437, 213860; DOI: https://doi.org/10.1016/j.ccr.2021.213860.
M. Koy, P. Bellotti, M. Das, F. Glorius, Nat. Catal., 2021, 4, 352; DOI: https://doi.org/10.1038/s41929-021-00607-z.
M. Watanabe, M. L. Thomas, S. Zhang, K. Ueno, T. Yasuda, K. Dokko, Chem. Rev., 2017, 117, 7190; DOI: https://doi.org/10.1021/acs.chemrev.6b00504.
H. A. Elwan, R. Thimmappa, M. Mamlouk, K. Scott, J. Power Sources, 2021, 510, 230371; DOI: https://doi.org/10.1016/j.jpowsour.2021.230371.
A. Ray, B. Saruhan, Materials, 2021, 14, 2942; DOI: https://doi.org/10.3390/ma14112942.
A. Nasr, A. Winkler, M. Tamm, Coord. Chem. Rev., 2016, 316, 68; DOI: https://doi.org/10.1016/j.ccr.2016.02.011.
E. Peris, Chem. Rev., 2018, 118, 9988; DOI: https://doi.org/10.1021/acs.chemrev.6b00695.
L. Benhamou, N. Vujkovic, V. César, H. Gornitzka, N. Lugan, G. Lavigne, Organometallics, 2010, 29, 2616; DOI: https://doi.org/10.1021/om1003607.
L. Benhamou, E. Chardon, G. Lavigne, S. Bellemin-Laponnaz, V. César, Chem. Rev., 2011, 111, 2705; DOI: https://doi.org/10.1021/cr100328e.
Y. Zhang, V. César, G. Storch, N. Lugan, G. Lavigne, Angew. Chem., Int. Ed., 2014, 53, 6482; DOI: https://doi.org/10.1002/anie.201402301.
Y. Zhang, G. Lavigne, N. Lugan, V. César, Chem. Eur. J., 2017, 23, 13792; DOI: https://doi.org/10.1002/chem.201702859.
V. V. Chesnokov, M. A. Shevchenko, S. B. Soliev, V. A. Tafeenko, V. M. Chernyshev, Russ. Chem. Bull., 2021, 70, 1281; DOI: https://doi.org/10.1007/s11172-021-3212-5.
A. Y. Chernenko, A. V. Astakhov, V. V. Kutyrev, E. G. Gordeev, J. V. Burykina, M. E. Minyaev, V. N. Khrustalev, V. M. Chernyshev, V. P. Ananikov, Inorg. Chem. Front., 2021, 8, 3382; DOI: https://doi.org/10.1039/D1QI00453K.
D. V. Pasyukov, A. Y. Chernenko, K. E. Shepelenko, V. V. Kutyrev, V. N. Khrustalev, V. M. Chernyshev, Mendeleev Commun., 2021, 31, 176; DOI: https://doi.org/10.1016/j.mencom.2021.03.010.
V. M. Chernyshev, O. V. Khazipov, M. A. Shevchenko, A. Y. Chernenko, A. V. Astakhov, D. B. Eremin, D. V. Pasyukov, A. S. Kashin, V. P. Ananikov, Chem. Sci., 2018, 9, 5564; DOI: https://doi.org/10.1039/c8sc01353e.
V. Karthik, I. A. Bhat, G. Anantharaman, Organometallics, 2013, 32, 7006; DOI: https://doi.org/10.1021/om400585b.
V. Karthik, V. Gupta, G. Anantharaman, Organometallics, 2014, 33, 6218; DOI: https://doi.org/10.1021/om5009023.
S. A. Rahaman, B. Roy, S. Mandal, S. Bandyopadhyay, Inorg. Chem., 2016, 55, 1069; DOI: https://doi.org/10.1021/acs.inorgchem.5b02104.
K. T. Potts, Chem. Rev., 1961, 61, 87; DOI: https://doi.org/10.1021/cr60210a001.
R. M. Shaker, ARKIVOC, 2006, 59; DOI: https://doi.org/10.3998/ark.5550190.0007.904.
D. Dixit, P. K. Verma, R. K. Marwaha, J. Iran. Chem. Soc., 2021, 18, 2535; DOI: https://doi.org/10.1007/s13738-021-02231-x.
F. Buccheri, G. Cusmano, M. Gruttadauria, R. Noto, G. Werber, J. Heterocycl. Chem., 1997, 34, 1447; DOI: https://doi.org/10.1002/jhet.5570340512.
E. S. H. E. Ashry, Y. E. Kilany, N. Rashed, H. Assafir, Advances in Heterocyclic Chemistry, Ed. A. R. Katritzky, Academic Press, 1999, 75, 79; DOI: https://doi.org/10.1016/S0065-2725(08)60984-8.
E. S. H. El Ashry, S. Nadeem, M. R. Shah, Y. E. Kilany, in Advances in Heterocyclic Chemistry, Ed. A. R. Katritzky, Academic Press, 2010, 101, 161; DOI: https://doi.org/10.1016/S0065-2725(10)01005-6.
F. Buccheri, G. Cusmano, R. Noto, R. Rainieri, G. Werber, J. Heterocycl. Chem., 1987, 24, 521; DOI: https://doi.org/10.1002/jhet.5570240243.
C. A. Montanari, J. P. B. Sandall, Y. Miyata, J. Miller, J. Chem. Soc., Perkin Trans. 2, 1994, 2571; DOI: https://doi.org/10.1039/P29940002571.
A. Echevarria, S. E. Galembeck, M. A. M. Maciel, J. Miller, C. A. Montanari, V. M. Rumjanek, A. M. Simas, J. B. P. Sandall, Heterocycl. Commun., 1995, 1, 129; DOI: https://doi.org/10.1515/HC.1995.1.2-3.129.
C. Soares de Oliveira, V. Dos Santos Falcão-Silva, J. P. Siqueira-Júnior, D. P. Harding, B. F. Lira, J. G. F. Lorenzo, J. M. Barbosa-Filho, P. Filgueiras de Athayde-Filho, Molecules, 2011, 16; DOI: https://doi.org/10.3390/molecules16032023.
M. G. Organ, S. Avola, I. Dubovyk, N. Hadei, E. A. B. Kantchev, C. J. O’Brien, C. Valente, Chem.—Eur. J., 2006, 12, 4749; DOI: https://doi.org/10.1002/chem.200600206.
C. J. O’Brien, E. A. B. Kantchev, C. Valente, N. Hadei, G. A. Chass, A. Lough, A. C. Hopkinson, M. G. Organ, Chem.—Eur. J., 2006, 12, 4743; DOI: https://doi.org/10.1002/chem.200600251.
M. G. Organ, M. Abdel-Hadi, S. Avola, I. Dubovyk, N. Hadei, E. A. B. Kantchev, C. J. O’Brien, M. Sayah, C. Valente, Chem.—Eur. J., 2008, 14, 2443; DOI: https://doi.org/10.1002/chem.200701621.
A. Y. Chernenko, A. V. Astakhov, D. V. Pasyukov, P. V. Dorovatovskii, Y. V. Zubavichus, V. N. Khrustalev, V. M. Chernyshev, Russ. Chem. Bull., 2018, 67, 79; DOI: https://doi.org/10.1007/s11172-018-2040-8.
S. G. Guillet, V. A. Voloshkin, M. Saab, M. Beliš, K. Van Hecke, F. Nahra, S. P. Nolan, Chem. Commun., 2020, 56, 5953; DOI: https://doi.org/10.1039/D0CC02262D.
E. S. Degtyareva, J. V. Burykina, A. N. Fakhrutdinov, E. G. Gordeev, V. N. Khrustalev, V. P. Ananikov, ACS Catal., 2015, 5, 7208; DOI: https://doi.org/10.1021/acscatal.5b01815.
O. V. Khazipov, M. A. Shevchenko, D. V. Pasyukov, A. Y. Chernenko, A. V. Astakhov, V. A. Tafeenko, V. M. Chernyshev, V. P. Ananikov, Catal. Sci. Technol., 2020, 10, 1228; DOI: https://doi.org/10.1039/C9CY02041A.
C. Dash, M. M. Shaikh, P. Ghosh, Eur. J. Inorg. Chem., 2009, 2009, 1608; DOI: https://doi.org/10.1002/ejic.200900115.
A. Kumar, M. K. Gangwar, A. P. Prakasham, D. Mhatre, A. C. Kalita, P. Ghosh, Ino rg. Chem., 2016, 55, 2882; DOI: https://doi.org/10.1021/acs.inorgchem.5b02727.
A. V. Astakhov, O. V. Khazipov, A. Y. Chernenko, D. V. Pasyukov, A. S. Kashin, E. G. Gordeev, V. N. Khrustalev, V. M. Chernyshev, V. P. Ananikov, Organometallics, 2017, 36, 1981; DOI: https://doi.org/10.1021/acs.organomet.7b00184.
R. A. Mekheimer, Y. R. Ibrahim, E. A. Ahmed, W. Frey, Tetrahedron, 2009, 65, 9843; DOI: https://doi.org/10.1016/j.tet.2009.09.082.
I. Nikovskiy, A. Polezhaev, V. Novikov, D. Aleshin, A. Pavlov, E. Saffiulina, R. Aysin, P. Dorovatovskii, L. Nodaraki, F. Tuna, Y. Nelyubina, Chem.—Eur. J., 2020, 26, 5629; DOI: https://doi.org/10.1002/chem.202000047.
L. Zhen, H. Fan, X. Wang, L. Jiang, Org. Lett., 2019, 21, 2106; DOI: https://doi.org/10.1021/acs.orglett.9b00383.
K. Sasse, Justus Liebigs Ann. Chem., 1970, 735, 158; DOI: https://doi.org/10.1002/jlac.19707350120.
K. N. Farrugia, D. Makuc, A. Podborska, K. Szaciłowski, J. Plavec, D. C. Magri, Org. Biomol. Chem., 2015, 13, 1662; DOI: https://doi.org/10.1039/C4OB02091J.
CrysAlisPro. Version 1.171.41. Rigaku Oxford Diffraction, 2021.
G. Sheldrick, Acta Crystallogr., Sect. A, 2015, 71, 3; DOI: https://doi.org/10.1107/S2053273314026370.
G. Sheldrick, Acta Crystallogr., Sect. C:, 2015, 71, 3; DOI: https://doi.org/10.1107/S2053229614024218.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339; DOI: https://doi.org/10.1107/S0021889808042726.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 5, pp. 993–1008, May, 2022.
The authors are grateful to Academician of the Russian Academy of Sciences V. P. Ananikov for fruitful discussion of the results of the work and valuable comments, to the “Nanotechnologies” Center of Collective Use of the Platov South-Russian State Polytechnic University, and to the Center of Collective Use of the N. D. Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences for conducting analytical experiments.
This work was carried out within the framework of the national project “Science and Universities” and was financially supported by the Ministry of Science and Higher Education of the Russian Federation (Project No. 075-03-2021-016/4) in the laboratory “New composite and functional materials with special properties”.
No human or animal subjects were used in this research.
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Pasyukov, D.V., Chernenko, A.Y., Lavrentev, I.V. et al. Dimroth rearrangement “thiadiazole-triazole”: synthesis and exploration of 3-sulfanyl-1,2,4-triazolium salts as NHC-proligands. Russ Chem Bull 71, 993–1008 (2022). https://doi.org/10.1007/s11172-022-3501-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3501-7