Abstract
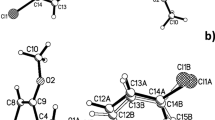
2,2-Dimethyl-3-[(4-methylphenyl)sulfonyl]-2,3-dihydro-1,3,2-benzoxazasilole was synthesized by the reaction of N-(2-hydroxyphenyl)-4-methylbenzenesulfonamide with dimethyl-dichlorosilane or N, N-bis(dimethylamino)dimethylsilane in 68 and 96% yield, respectively. The structure of the new compound was assigned using NMR and IR spectroscopy and confirmed by X-ray diffraction. In the crystal, the silole molecules are linked to each other by short O⋯H-C contacts (∼2.6 Å) between the oxygen atoms of the bicyclic moiety and the methyl hydrogen atoms of adjacent molecules.
Similar content being viewed by others
References
S. Bhadra, H. Yamamoto, Chem. Rev., 2018, 118, 3391; DOI: https://doi.org/10.1021/acs.chemrev.7b00514.
C. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes, M. Schnürch, Chem. Soc. Rev., 2018, 47, 6603; DOI: https://doi.org/10.1039/c8cs00201k.
P. Ribiere, V. Declerck, J. Martinez, F. Lamaty, Chem. Rev., 2006, 106, 2249; DOI: https://doi.org/10.1021/cr0300587.
J. D. Wilden, J. Chem. Res., 2010, 34, 541; DOI: https://doi.org/10.3184/030823410X12857514635822.
J. A. Halfen, Curr. Org. Chem., 2005, 9, 657; DOI: https://doi.org/10.2174/1385272053765024.
H. Yang, R. G. Carter, Synlett., 2010, 2827; DOI: https://doi.org/10.1055/s-0030-1259020.
F. Carta, C. T. Supuran, A. Scozzafava, Future Med. Chem., 2014, 6, 1149; DOI: https://doi.org/10.4155/fmc.14.68.
T. V. Wani, S. Bua, P. S. Khude, A. H. Chowdhary, C. T. Supuran, M. P. Toraskar, J. Enzym. Inhib. Med. Chem., 2018, 33, 962; DOI: https://doi.org/10.1080/14756366.2018.1471475.
S. S. A. Shah, G. Rivera, M. Ashfaq, Mini Rev. Med. Chem., 2013, 13, 70; DOI: https://doi.org/10.2174/1389557511307010070.
R. Kh. Bagautdinova, L. I. Vagapova, A. V. Smolobochkin, A. S. Gazizov, A. R. Burilov, M. A. Pudovik, A. D. Voloshina, Mendeleev Commun., 2019, 29, 686; DOI: https://doi.org/10.1016/j.mencom.2019.11.028.
I. Melnikova, Nat. Rev. Drug Discov., 2005, 4, 453; DOI: https://doi.org/10.1038/nrd1755.
A. Bertolini, A. Ottani, M. Sandrini, Curr. Med. Chem., 2002, 9, 1033; DOI: https://doi.org/10.2174/0929867024606650.
J. Dong, Y. Wang, Q. Xiang, X. Lv, W. Weng, Q. Zeng, Adv. Synth. Catal., 2013, 355, 692; DOI: https://doi.org/10.1002/adsc.201200752.
D.-W. Zhang, X. Zhao, J.-L. Hou, Z.-T. Li, Chem. Rev., 2012, 112, 5271; DOI: https://doi.org/10.1021/cr300116k.
N. N. Farshori, A. Ahmad, A. U. Khan, A. Rauf, Eur. J. Med. Chem., 2011, 46, 1433; DOI: https://doi.org/10.1016/j.ejmech.2011.01.070.
P. Panini, R. G. Gonnade, D. Chopra, New J. Chem., 2016, 40, 4981; DOI: https://doi.org/10.1039/C5NJ03211C.
H.-Y. Lee, S.-L. Pan, M.-C. Su, Y.-M. Liu, C.-C. Kuo, Y.-T. Chang, J.-S. Wu, C.-Y. Nien, S. Mehndiratta, C.-Y. Chang, S.-Y. Wu, M.-J. Lai, J.-Y. Chang, J.-P. Liou, J. Med. Chem., 2013, 56, 8008; DOI: https://doi.org/10.1021/jm4011115-8018.
J. Kim, P. Chun, H. R. Moon, Bull. Korean Chem. Soc., 2013, 34, 1487; DOI:https://doi.org/10.5012/BKCS.2013.34.5.1487.
R. Mahesh, V. L. Nayak, K. S. Babu, S. Riyaz, T. B. Shaik, G. B. Kumar, P. L. Mallipeddi, C. R. Reddy, K. C. Shekar, J. Jose, N. Nagesh, A. Kamal, Chem. Med. Chem., 2017, 12, 678; DOI: https://doi.org/10.1002/cmdc.201600643.
J. Mortier, C. Rakers, M. Bermudez, M. S. Murgueitio, S. Riniker, G. Wolber, Drug Discov. Today, 2015, 20, 686; DOI: https://doi.org/10.1016/j.drudis.2015.01.003.
H. Zhao, A. Caflisch, Eur. J. Med. Chem., 2015, 91, 4; DOI: https://doi.org/10.1016/j.ejmech.2014.08.004-14.
Structure-Based Drug Discovery, Ed. L. W. Tari, Springer, New York, 2012, 385 p.; DOI: https://doi.org/10.1007/978-1-61779-520-6.
Noncovalent Forces, Ed. S. Scheiner, Springer, Cham Heidelberg, 2015, 529 p.; DOI: https://doi.org/10.1007/978-3-319-14163-3.
E. Lukevics, S. Germane, I. Segal, A. Zablotskaya, Chem. Heterocycl. Compd., 1997, 33, 234; DOI: https://doi.org/10.1007/BF02256766.
M. V. Kashutina, S. L. Ioffe, V. A. Tartakovskii, Russ. Chem. Rev., 1975, 44, 1620; DOI: https://doi.org/10.1070/RC1975v044n09ABEH002373.
M. D. Mizhiritskii, Yu. A. Yuzhelevskii, Russ. Chem. Rev., 1987, 56, 355; DOI: https://doi.org/10.1070/RC1987v056n04ABEH003276.
G. Look, Silylating Agents, Buchs, Fluka Chemie AG, 1995, 43 p.
G. A. Patani, E. J. LaVoie, Chem. Rev., 1996, 96, 3147; DOI: https://doi.org/10.1021/cr950066q.
N. A. Meanwell, J. Med. Chem., 2011, 54, 2529; DOI: https://doi.org/10.1021/jm1013693-2591.
J. S. Mills, G. A. Showell, Expert Opin. Invest. Drugs, 2004, 13, 1149; DOI: https://doi.org/10.1517/13543784.13.9.1149.
A. K. Franz, S. O. Wilson, J. Med. Chem., 2013, 56, 388; DOI: https://doi.org/40510.1021/jm3010114.
A. Yu. Nikonov, I. V. Sterkhova, V. Yu. Serykh, N. A. Kolyvanov, N. F. Lazareva, J. Mol. Struct., 2019, 1198, 126782; DOI: https://doi.org/10.1016/j.molstruc.2019.07.029.
N. F. Lazareva, A. Yu. Nikonov, N. N. Chipanina, L. P. Oznobikhina, I. V. Sterkhova, A. I. Albanov, J. Organomet. Chem., 2017, 846, 88; DOI: https://doi.org/10.1016/j.jorganchem.2017.05.061.
Y. Tanabe, T. Misaki, M. Kurihara, A. Iida, Y. Nishii, Chem. Commun., 2002, 1628; DOI: https://doi.org/10.1039/b203783c.
B. Minkovich, I. Ruderfer, A. Kaushansky, D. Bravo-Zhivotovskii, Y. Apeloig, Angew. Chem., Int. Ed., 2018, 57, 13261; DOI: https://doi.org/10.1002/anie.201807027.
E. K. J. Lui, J. W. Brandt, L. L. Schafer, J. Am. Chem. Soc., 2018, 140, 4973; DOI: https://doi.org/10.1021/jacs.7b13783.
G. Glatz, T. Schmalz, T. Kraus, F. Haarmann, G. Motz, R. Kempe, Chem. Eur. J., 2010, 16, 4231; DOI: https://doi.org/10.1002/chem.200902836.
E. Kroke, Y.-L. Li, C. Konetschny, E. Lecomte, C. Fasel, R. Riedel, Mater. Sci. (Engl.) R: Reports, 2000, 26, 97; DOI: https://doi.org/10.1016/S0927-796X(00)00008-5.
G. M. Sheldrick, Acta Crystallogr., 2008, D64, 112; DOI: https://doi.org/10.1107/S010876730704393.
V. Passarelli, F. Benetollo, P. Zanella, G. Carta, G. Rossetto, Dalton Trans., 2003, 1411; DOI: https://doi.org/10.1039/B212705A.
W. L. F. Armarego, C. L. L. Chai, Purification of Laboratory Chemicals, 6th Ed., Elsevier, 2009, 760 c.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was financially supported by the Russian Foundation for Basic Research (Project No. 18-33-00368-mol_a). The main results were obtained using equipment of the Baikal Analytical Center for Collective Use of the Siberian Branch of the Russian Academy of Sciences.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 2, pp. 386–390, February, 2021.
Rights and permissions
About this article
Cite this article
Nikonov, A.Y., Sterkhova, I.V. & Lazareva, N.F. 2,2-Dimethyl-3-[(4-methylphenyl)sulfonyl]-2,3-dihydro-1,3,2-benzoxazasilole: synthesis, properties, and structure. Russ Chem Bull 70, 386–390 (2021). https://doi.org/10.1007/s11172-021-3097-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3097-3