Abstract
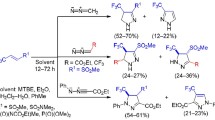
The peculiarities of the three-component cyclization of ethyl 4,4,4-trifluoroacetoacetate and 1,2-ethanediamines with 3-methylbutan-2-one into hexahydroimidazo[1,2-a]pyridin-5-ones were studied. The reactions proceeded under mild conditions. The use of methyl ketone with a bulky isopropyl substituent increased the stereoselectivity of the transformations, but the reaction with 1,2-diaminopropane was accompanied by the formation 4-[(1-ammoniopropan-2-yl)amino]-1,1,1-trifluoro-4-oxobut-2-en-2-olate as a by-product.
Similar content being viewed by others
References
Multicomponent Reactions, Eds J. P. Zhu, H. Bienayme, Wiley-VCH Weinheim, Germany, 2005, 468 pp.
A. Domling, Chem. Rev., 2006, 106, 17; DOI: https://doi.org/10.1021/cr0505728.
B. Ganem, Acc. Chem. Res., 2009, 42, 463; DOI: https://doi.org/10.1021/ar800214s.
A. Domling, W. Wang, K. Wang, Chem. Rev., 2012, 112, 3083; DOI: https://doi.org/10.1021/cr100233r.
I. Ugi, R. Meyr, Angew Chem., 1958, 70, 702.
M.G. Passerini, Chim. Ital., 1921, 51, 126.
A. Strecker, Liebigs Ann. Chem., 1850, 75, 27.
A. Hantzsch, Ber. Dtsch. Chem. Ges., 1890, 23, 1474.
P. Biginelli, Chem. Ber., 1891, 24, 1317.
J. Liu, J. Li, L. Zhang, L. Song, M. Zhang, W. Cao, S. Zhu, H. Deng, M. Shao, Tetrahedron Lett., 2012, 53, 2469; DOI: https://doi.org/10.1016/j.tetlet.2012.03.023.
W. Wang, J. Li, L. Zhang, L. Song, M. Zhang, W. Cao, H. Deng, M. Shao, Synthesis, 2012, 44, 1686; DOI: https://doi.org/10.1055/s-0031-1289761.
K. Karnakar, K. Ramesh, K. H. V. Reddy, B. S. P. Anil Kumar, J. B. Nanubonula, Y. V. D. Nageswar, New J. Chem., 2015, 39, 8978; DOI: https://doi.org/10.1039/C5NJ01448D.
N. N. Gibadullina, D. R. Latypova, R. A. Novikov, Y. V. Tomilov, V. A. Dokicheva, Arkivoc, 2017, 222; DOI: https://doi.org/10.24820/ark.5550190.p010.003.
L. Zhou, F. Yuan, Y. Zhou, W. Duan, M. Zhang, H. Deng, L. Song, Tetrahedron, 2018, 3761; DOI: https://doi.org/10.1016/j.tet.2018.05.059.
J. D. Bhatt, T. S. Patel, C. J. Chudasama, K. D. Patel, ChemistrySelect, 2018, 3, 3632; DOI: https://doi.org/10.1002/slct.201702285.
C. Dayakar, B. C. Raju, ChemistrySelect, 2018, 3, 9388; DOI: https://doi.org/10.1002/slct.201801430.
X.-X. Du, Q.-X. Zi, Y.-M. Wu, Y. Jin, J. Lin, S.-J. Yan, Green Chem., 2019, 21, 1505; DOI: https://doi.org/10.1039/C8GC03698E.
W. K. Hagmann, J. Med. Chem., 2008, 51, 4359; DOI: https://doi.org/10.1021/jm800219f.
S. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320; DOI: https://doi.org/10.1039/B610213C.
J. Wang, M. Sánchez-Roselló, J. L. Acena, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok, H. Liu, Chem. Rev., 2014, 114, 2432; DOI: https://doi.org/10.1021/cr4002879.
T. Hiyama, Organo fluorine Compounds: Chemistry and Applications, Ed. H. Yamamoto, Springer-Verlag, Berlin, 2000.
Fluorine in Medicinal Chemistry and Chemical Biology, Ed. I. Ojima, Wiley-Blackwell, Chichester, 2009.
M. V. Pryadeina, Ya. V. Burgart, V. I. Saloutin, M. I. Kodess, E. N. Ulomskii, V. L. Rusinov, Russ. J. Org. Chem., 2004, 40, 902; DOI: https://doi.org/10.1023/B:RUJO.0000044558.47152.65.
M. V. Pryadeina, Ya. V. Burgart, V. I. Saloutin, O. N. Chupakhin, Mendeleev Commun., 2008, 18, 276; DOI: https://doi.org/10.1016/j.mencom.2008.09.017.
M. V. Goryaeva, Ya. V. Burgart, V. I. Saloutin, E. V. Sadchikova, E. N. Ulomskii, Heterocycles, 2009, 78, 435; DOI: https://doi.org/10.3987/COM-08-11524.
M. V. Goryaeva, Ya. V. Burgart, V. I. Saloutin, J. Fluorine Chem., 2013, 147, 15; DOI: https://doi.org/10.1016/j.jfluchem.2013.01.005.
Yu. S. Kudyakova, D. N. Bazhin, M. V. Goryaeva, Ya. V. Burgart, V. I. Saloutin, Russ. Chem. Rev., 2014, 83, 120; DOI: https://doi.org/10.1070/RC2014v083n02ABEH004388.
M. V. Goryaeva, Ya. V. Burgart, M. A. Ezhikova, M. I. Kodess, V. I. Saloutin, Beilstein J. Org. Chem., 2015, 11, 385; DOI: https://doi.org/10.3762/bjoc.11.44.
V. I. Saloutin, Yu. S. Kudyakova, M. V. Goryaeva, Ya. V. Burgart, O. N. Chupakhin, Pure Appl. Chem., 2017, 89, 1209; DOI: https://doi.org/10.1515/pac-2016-1015.
V. I. Saloutin, Ya. V. Burgart, O. G. Kuzueva, C. O. Kappe, O. N. Chupakhin, J. Fluor. Chem., 2000, 103, 17; DOI: https://doi.org/10.1016/S0022-1139(99)00216-X.
M. V. Goryaeva, Ya. V. Burgart, Yu. S. Kudyakova, M. A. Ezhikova, M. I. Kodess, P. A. Slepukhin, V. I. Saloutin, Eur. J. Org. Chem., 2015, 2015, 6306; DOI: https://doi.org/10.1002/ejoc.201500822.
M. V. Goryaeva, Ya. V. Burgart, Yu. S. Kudyakova, M. A. Ezhikova, M. I. Kodess, V. I. Saloutin, Eur. J. Org. Chem., 2017, 3986; DOI: https://doi.org/10.1002/ejoc.201700683.
G. M. Sheldrick, Acta Crystallogr., Sect. A: Foundations of Crystallography, 2008, 64, 112; DOI: https://doi.org/10.1107/S0108767307043930.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was financially supported by the Russian Foundation for Basic Research (Project No. 18-03-00342) and in the framework of the Russian state assignment (Topic No. AAAA-A19-119011790132-7). Analytical studies were carried out using the equipment of the Centre for Joint Use “Spectroscopy and Analysis of Organic Compounds” at the I. Ya. Postovsky Institute of Organic Synthesis of the Ural Branch of the Russian Academy of Sciences.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 11, pp. 2163—2166, November, 2020.
Rights and permissions
About this article
Cite this article
Goryaeva, M.V., Kushch, S.O., Burgart, Y.V. et al. Peculiarities of three-component cyclization of ethyl 4,4,4-trifluoroacetoacetate and 1,2-ethanediamines with 3-methylbutan-2-one. Russ Chem Bull 69, 2163–2166 (2020). https://doi.org/10.1007/s11172-020-3016-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-020-3016-z