Abstract
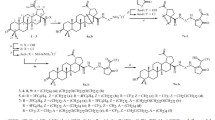
A new promising conjugate of betulinic acid with N-acetyl-d-galactosamine was synthesized by the simple reaction sequence: esterification and copper-catalyzed azide-alkyne cycloaddition. The obtained glycoderivative exhibited high activity against hepatocarcinoma cell lines in vitro, selectivity of cytotoxic action, and excellent binding to the asialoglycoprotein receptor (ASGPR) of hepatocytes. Its affinity to the ASGPR was established by surface plasmon resonance spectroscopy and confirmed by molecular docking in silico. An original approach was proposed to enhance the cytotoxic properties of C-28 betulinic esters by introducing a hemioxalate fragment bearing free carboxyl group into the C(3) position of ring A.
Similar content being viewed by others
References
R. Dutta, R. I. Mahato, Pharmacol. Ther., 2017, 173, 106.
L. A. Torre, F. Bray, R. L. Siegel, J. Ferlay, J. Lortet-Tieulent, A. Jemal, CA Cancer J. Clin., 2015, 65, 87.
X. Zhang, H. L. H. Ng, A. Lu, C. Lin, L. Zhou, G. Lin, Y. Zhang, Z. Yang, H. Zhang, Nanomedicine, 2016, 12, 853.
M. S. Butler, A. A. Robertson, M. A. Cooper, Nat. Prod. Rep., 2014, 31, 1612.
D. J. Newman, G. M. Cragg, J. Nat. Prod., 2016, 79, 629.
M. Ali-Seyed, I. Jantan, K. Vijayaraghavan, S. N. A. Bukhari, Chem. Biol. DrugDes., 2016, 87, 517.
J. Yang, B. Qiu, X. Li, H. Zhang, W. Liu, Toxicol. Lett., 2015, 238, 1.
R. Csuk, Expert Opin. Ther. Patents, 2014, 24, 913.
S. C. Jonnalagadda, M. A. Corsello, C. E. Sleet, Anti-Cancer Agents Med. Chem., 2013, 13, 1477.
D. M. Zhang, H. G. Xu, L. Wang, Y. J. Li, P. H. Sun, X. M. Wu, G. J. Wang, W. M. Chen, W. C. Ye, Med. Res. Rev., 2015, 35, 1127.
I. Mierina, R. Vilskersts, M. Turks, Curr. Med. Chem., 2019, 25, 1.
M. Zhou, R. H. Zhang, M. Wang, G. B. Xu, S. G. Liao, Eur. J. Med. Chem., 2017, 131, 222.
D. E. Large, J. R. Soucy, J. Hebert, D. T. Auguste, Adv. Therap., 2019, 2, 1800091.
Ya. A. Ivanenkov, S. Yu. Maklakova, E. K. Beloglazkina, N. V. Zyk, A. G. Nazarenko, A. G. Tonevitsky, V. E. Kotelianski, A. G. Majouga, Russ. Chem. Rev., 2017, 86, 750.
A. A. D’Souza, P. V. Devarajan, J. Control. Release, 2015, 203, 126.
E. Yu. Yamansarov, D. A. Skvortsov, A. V. Lopuhov, S. V. Kovalev, S. A. Evteev, R. A. Petrov, N. L. Klyachko, N. V. Zyk, E. K. Beloglazkina, Ya. A. Ivanenkov, A. G. Majouga, Russ. Chem. Bull., 2019, 68, 2331.
M. Ortega-Munoz, F. Rodriguez-Serrano, E. De Los Reyes-Berbel, N. Mut-Salud, F. Hernandez-Mateo, A. Rodriguez-López, J. M. Garrido, F. J. Lopez-Jaramillo, F. Santoyo-Gonzalez, ACS Omega, 2018, 3, 11455.
D. A. Nedopekina, R. R. Gubaidullin, V. N. Odinokov, P. V. Maximchik, B. Zhivotovsky, Y. P. Bel’skii, V. A. Khazanov, A. V. Manuylova, V. Gogvadze, A. Yu. Spivak, Med. Chem. Comm., 2017, 8, 1934.
E. Yu. Yamansarov, I. V. Saltykova, S. V. Kovalev, R. A. Petrov, D. O. Shkilr, E. I. Seleznev, E. K. Beloglazkina, A. G. Majouga, Russ. Chem. Bull, 2019, 4, 855.
D. Bhunia, P. M. Pallavi, S. R. Bonam, S. A. Reddy, Y. Verma, M. S. K. Halmuthur, Arch. Pharm., 2015, 348, 689.
P. Zhu, Y. Bi, J. Xu, Z. Li, J. Liu, L. Zhang, W Ye, X. Wu, Bioorg. Med. Chem. Lett., 2009, 19, 6966.
S. B. Salunke, N. Seshu Babu, C.-T. Chen, Chem. Comm., 2011, 47, 10440.
M. S. Singh, S. Chowdhury, S. Koley, Tetrahedron, 2016, 72, 5257.
J. K. Nair, J. L. S. Willoughby, A. Chan, K. Charisse, Md. R. Alam, Q. Wang, M. Hoekstra, P. Kandasamy, A. V. Kel’in, S. Milstein, N. Taneja, J. O’Shea, S. Shaikh, L. Zhang, R. J. van der Sluis, M. E. Jung, A. Akinc, R. Hutabarat, S. Kuchimanchi, K. Fitzgerald, T. Zimmermann, T. J. C. van Berkel, M. A. Maier, K. G. Rajeev, M. Manoharan, J. Am. Chem. Soc, 2014, 136, 16958.
T. Mosmann, J. Immunol. Methods, 1983, 65, 55.
M. Tanowitz, L. Hettrick, A. Revenko, G. A. Kinberger, T. P. Prakash, P. P. Seth, Nucleic Acids Res., 2017, 45, 12388.
J. Hou, X. Liu, J. Shen, G. Zhao, P. G. Wang, Expert Opin. Drug Discov., 2012, 7, 489.
W I. Weis, M. E. Taylor, K. Drickamer, Immunol. Rev., 1998, 163, 19.
X. Huang, J. C. Leroux, B. Castagner, Bioconjugate Chem., 2017, 28, 283.
C. A. Sanhueza, M. M. Baksh, B. Thuma, M. D. Roy, S. Dutta, C. Prévffle, B. A. Chrunyk, K. Beaumont, R. Dullea, M. Ammirati, S. Liu, D. Gebhard, J. E. Finley, C. T. Salatto, A. King-Ahmad, I. Stock, K. Atkinson, B. Reidich, W. Lin, R. Kumar, M. Tu, E. Menhaji-Klotz, D. A. Price, S. Liras, M. G. Finn, V. Mascitti, J. Am. Chem. Soc., 2017, 139, 3528.
E. F. Pettersen, T. D. Goddard, C. C. Huang, G. S. Couch, D. M. Greenblatt, E. C. Meng, T. E. Ferrin, J. Comput. Chem., 2004, 25, 1605.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences V. V. Lunin on the occasion of his 80th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 0158–0163, January, 2020.
Rights and permissions
About this article
Cite this article
Olshanova, A.S., Yamansarov, E.Y., Seleznev, E.I. et al. Synthesis of a new betulinic acid glycoconjugate with N-acetyl-d-galactosamine for the targeted delivery to hepatocellular carcinoma cells. Russ Chem Bull 69, 158–163 (2020). https://doi.org/10.1007/s11172-020-2737-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-020-2737-3