Abstract
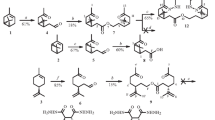
Six optically active macroheterocycles bearing ester and dihydrazide moieties were synthesized from available natural (-)-α-pinene through the intermediate keto acid, (31R,33R)- 1-hydroxy-32,32-dimethyl-1,4-dioxopentaphane, using the [2+1] reaction of this keto acid with di- or triethylene glycols and the [1+1] condensation of the resulting α,ω-diketo diesters with dicarboxylic acid dihydrazides in the key steps.
Similar content being viewed by others
References
G. Yu. Ishmuratov, M. P. Yakovleva, G. R. Mingaleeva, A. G. Tolstikov, Makrogeterotsikly [Macroheterocycles], 2011, 4, 270 (in Russian).
G. Yu. Ishmuratov, M. P. Yakovleva, V. A. Vydrina, O. O. Shakhanova, N. M. Ishmuratova, A. G. Tolstikov, Makrogeterotsikly [Macroheterocycles], 2012, 5, 212 (in Russian).
G. Yu. Ishmuratov, M. P. Yakovleva, V. A. Vydrina, M. A. Shutova, N. M. Ishmuratova, A. G. Tolstikov, Makrogeterotsikly [Macroheterocycles], 2017, 10, 345 (in Russian).
S. V. Larionov, L. I. Myachina, L. A. Sheludyakova, E. G. Boguslavskii, S. N. Bizyaev, A. V. Tkachev, Russ. J. Inorg. Chem., 2007, 52, 42.
S. V. Larionov, A. V. Tkachev, L. I. Myachina, S. N. Bizyaev, L. A. Glinskaya, R. F. Klevtsova, Dokl. Chem., 2006, 411, 206.
M. C. de la Torre, M. Asenjo, P. Ramirez-Lopez, M. A. Sierra, Eur. J. Org. Chem., 2015, 1054.
E. E. Anagnostaki, Z. L. Zografos, Org. Lett., 2013, 15, 152.
S. C. Philkhana, B. Seetharamsingh, Y. B. Dangat, K. Vanka, D. S. Reddy, Chem. Commun., 2013, 49, 3342.
F. Bouazza, B. Renoux, C. Bachmann, J.-P. Gesson, Org. Lett., 2003, 5, 4049.
D. V. Belykh, E. V. Buravlev, I. Yu. Chukicheva, I. S. Tarabukina, O. G. Shevchenko, S. N. Plyusnina, A. V. Kutchin, Russ. J. Bioorg. Chem., 2012, 38, 558.
E. V. Buravlev, I. Yu. Chukicheva, D. V. Belykh, A. V. Kutchin, Chem. Nat. Compd., 2007, 43, 678.
K. Nakashima, Y. Kondo, K. Yoshimasu, M. Sono, M. Tori, Nat. Prod. Commun., 2015, 10, 551.
G. Y. Ishmuratov, G. R. Mingaleeva, M. P. Yakovleva, O. O. Shakhanova, R. R. Muslukhov, A. G. Tolstikov, Russ. J. Org. Chem., 2011, 47, 1416.
G. Yu. Ishmuratov, V. A. Vydrina, K. S. Denisova, M. P. Yakovleva, R. R. Gazetdinov, E. M. Vyrypaev, A. G. Tolstikov, Chem. Nat. Compd., 2017, 53, 63.
G. Y. Ishmuratov, Yu. V. Legostaeva, L. R. Garifullina, L. P. Botsman, Z. I. Idrisova, R. R. Muslukhov, N. M. Ishmuratova, G. A. Tolstikov, Russ. J. Org. Chem., 2013, 49, 1409.
Weygand-Hilgetag, Organisch-Chemische Experimentierkunst, J. A. Barth Verlag, Leipzig, 1964.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 7, pp. 1445–1450, July, 2019.
Rights and permissions
About this article
Cite this article
Yakovleva, M.P., Mingaleeva, G.R., Denisova, K.S. et al. Synthesis of optically active macrolides bearing di- and triethylene glycol and dicarboxylic acid hydrazide moieties from (-)-α-pinene. Russ Chem Bull 68, 1445–1450 (2019). https://doi.org/10.1007/s11172-019-2575-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-019-2575-3