Abstract
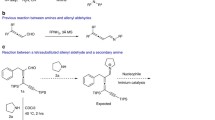
The N(1)—N(1), N(2)—N(2), and N(1)—N(2) regioisomers of 1,2-bis[(prop-2-yn-1-yl)-1H-tetrazol-5-yl]ethane were first synthesized by alkylation of 1,2-bis(1H-tetrazol-5-yl)ethane with propargyl bromide. The peculiarities of the crystal structure of 1,2-bis[1-(prop-2-yn-1-yl)-1H-tetrazol-5-yl]ethane were evaluated by X-ray diffraction analysis. This compound is readily underwent Cu-catalyzed [3+2] cycloaddition with p-tolyl azide, p-nitrophenyl azide, and benzyl azide to give heterocyclic assembles bearing 1,2,3-triazole and tetrazole cycles. Catalyst-free [3+2] cycloadditions of 1,2-bis[1-(prop-2-yn-1-yl)-1H-tetrazol-5-yl]ethane and the mixtures of the N(1)—N(1), N(2)—N(2), N(1)—N(2) regioisomers with poly(glycidyl azide) oligomers resulted in 1,2,3-triazole cycles and crosslinking of the polymer chains.
Similar content being viewed by others
References
R. E. Trifonov, V. A. Ostrovskii, Russ. J. Org. Chem. (Engl. Transl.), 2006, 42, 1585 [Zh. Org. Khim., 2006, 42, 1599].
V. A. Ostrovskii, R. E. Trifonov, E. A. Popova, Russ. Chem. Bull. (Int. Ed.), 2012, 61, 768 [Izv. Akad. Nauk, Ser. Khim., 2012, 765].
G. L. Rusinov, R. I. Ishmetova, M. A. Kravchenko, D. G. Beresnev, V. G. Kitaeva, E. I. Tolstykh, V. A. Sokolov, O. N. Chupakhin, Pharm. Chem. J. (Engl. Transl.), 2000, 34, 416 [Khim.-Farm. Zh., 2000, 34, No. 8, 23].
L. I. Vereschagin, O. N. Verkhosina, F. A. Pokatilov, V. N. Kizhnyaev, Russ. J. Org. Chem. (Engl. Transl.), 2007, 43, 1575 [Zh. Org. Khim., 2007, 43, 1577].
S. E. Ivanova, G. I. Koldobskii, V. A. Ostrovskii, Chem. Heterocycl. Compd. (Engl. Transl.), 1993, 29, 770 [Khim. Geterotsikl. Soedin., 1993, 29, 770].
W.-F. Zhu, X.-F. Zhou, Acta Cryst., 2008, E64, 1478.
V. N. Kizhnyaev, F. A. Pokatilov, L. I. Vereshchagin, Polymer Science. Ser. A (Engl. Transl.), 2007, 49, 28 [Vysokomol. Soedin., Ser. A, 2007, 49, 36].
V. A. Ostrovskii, P. A. Aleshunin, V. Yu. Zubarev, E. A. Popova, Yu. N. Pavlyukova, E. A. Shumilova, P. E. Trifonov, T. V. Artamonova, Russ. J. Org. Chem. (Engl. Transl.), 2010, 46, 1678 [Zh. Org. Khim., 2010, 46, 1671].
K. Nakanishi, Infrared Absorption Spectroscopy, Holden-Day, Inc., San Francisco, 1962, pp. 233.
F. R. Benson, in Heterocyclic Compounds, Vol. 8, Ed. R. C. Elderfield, J. Wiley and Sons, New York—London—Sydney, 1967, Ch. 1.
G. I. Koldobskii, V. A. Ostrovskii, V. S. Poplavskii, Chem. Heterocycl. Compd. (Engl. Transl.), 1981, 17, 965 [Khim. Geterotsikl. Soedin., 1981, 1299].
P. N. Gaponik, O. A. Ivashkevich, O. N. Bubel´, M. M. Degtyarik, V. N. Naumenko, Theor. Exp. Chem. (Engl. Transl.), 1989, 25, 30 [Teor. eksp. khim., 1989, 25, 33].
J. H. Boyer, in Heterocyclic Compounds, Vol. 7, Ed. R. C. Elderfield, J. Wiley and Sons, New York, 1961.
Jung-Ah Shin, Yeong-Gweon Lim, Kyung-Hee Lee, Bull. Korean Chem. Soc., 2011, 32, 547.
V. Yu. Zubarev, R. E. Trifonov, V. V. Poborchii, V. A. Ostrovskii, Chem. Heterocycl. Compd. (Engl. Transl.), 2006, 42, 469 [Khim. Geterotsikl. Soedin., 2006, 42, 469].
J. McNulty, K. Keskar, Eur. J. Org. Chem., 2012, 5462.
V. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596.
S. Brochu, G. Ampleman, Macromolecules, 1996, 29, 5539.
G. M. Sheldrick, Acta Crystallogr., Sect. A, 2008, 64, 112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences O. G. Sinyashin on the occasion of his 60th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 5, pp. 1268–1271, May, 2016.
Rights and permissions
About this article
Cite this article
Ishmetova, R.I., Yachevskii, D.S., Ignatenko, N.K. et al. Terminal bis-acetylenes derived from 1,2-bis(1H-tetrazol-5-yl)ethane. Russ Chem Bull 65, 1268–1271 (2016). https://doi.org/10.1007/s11172-016-1446-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-016-1446-4