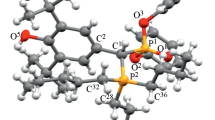
Abstract
Methods for the synthesis of quaternary phosphonium salts based on 3-hydroxypyridine and 4-deoxypyridoxine were developed. Some of obtained compounds possess high antibacterial and antitumor activity in vitro.
Similar content being viewed by others
References
C. L. Zhu, Angew. Chem., Int. Ed., 2011, 50, 5869.
D. Uraguchi, Synlett., 2011, 9, 1265.
H. Fujioka, Angew. Chem., Int. Ed., 2011, 50, 12232.
J. Emsley, The Chemistry of Phosphorus, Harper and Row, London, 1976, 560 p.
E. Zbiral, Organophosphorus Reagents in Organic Synthesis, Academic Press, London, 1979, 608 pp.
D. Cao, Z. Chai, J. Zhang, Z. Ye, H. Xiao, H. Wang, J. Chen, X. Wua, G. Zhao, Chem. Commun., 2013, 49, 5972.
H. Lund, J. Harloff, A. Schulz, A. Villinger, Z. Anorg. Allg. Chem., 2013, 639, 754.
A. M. A. Dias, S. Marceneiro, M. E. M. Braga, J. F. J. Coelho, A. G. M. Ferreira, P. N. Simxes, H. I. M. Veiga, L. C. Tome, I. M. Marrucho, J. M. S. S. Esperanca, A. A. Matias, C. M. M. Duarte, L. P. N. Rebelo, H. C. de Sousa, Acta Biomater., 2012, 8, 1366.
C. J. Meyer, M. Vogt, J. P. Catalino, B. L. Ashfeld, Synlett., 2013, 24, 1428.
J. J. Tindale, K. L. Mouland, P. J. Ragogna, J. Mol. Liq., 2010, 152, 14.
H. Bestmann, K. H. Büchel, J. Houben, E. Müller, M. Regitz, T. Weyl, Organische Phosphorverbindungen 1, Thieme, Stuttgart, 1982, 972 pp.
D. Purdela, R. Volceanu, Chimia compusilor organici ai fosforului si ai acizilor lui, Editura Academiei Republicii Socialiste Romania, Romania, 1965, 539.
L. Kasukhin, V. Brovarets, O. Smolii, V. Kurg, L. Budnik, B. S. Drach, J. Gen. Chem. (Engl. Transl.), 1991, 61, 2679 [Zh. Obshch. Khim, 1991, 61, 2679].
P. McAllister, M. Dotson, S. Grim, G. Hillman, J. Med. Chem., 1980, 23, 862.
D. C. Rideout, T. Calogeropoulou, J. S. Jaworski, R. J. Dagnino, M. R. McCarthy, Anti-Cancer Drug Design., 1989, 4, 265.
G. V. Romanov, O. K. Pozdeev, G. Kh. Gil´manova, T. Ya. Ryzhikova, E. P. Semkina, Pharmaceut. Chem. J., 1990, 24, 414.
US Pat. 4297487, 1981; http://patft.uspto.gov/netacgi/nphParser?Sect2=PTO1&Sect2=HITOFF&p=1&u=/netahtml/PTO/search-bool.html&r=1&f=G&1=50&d= PALL&RefSrch=yes&Query=PN/4297487.
I. Galkina, Yu. Bakhtiyarova, V. Andriyashin, V. Galkin, R. Cherkasov, Phosph. Sulf. Silicon, 2013, 188, 15.
A. Kanazava, T. Ikeda, T. Endo, Antimicrob. Agents Chemother., 1994, 38, 945.
JP Pat. 2005060332, 2005; https://www19.j-platpat.inpit.go.jp/PA1/cgi-bin/PA1DETAIL?MaxCount=1000&PageCount=1000&SearchType=0&TempName=wL-adya&MaxPage= 1&DispPage=1+1000&HitCount=1&ResultId= I00222005801&CookieId=2&DetailPage=1&Language= ENG&Reserve1=DetailPaging&Reserve2=Ky2ZA2759_ FS086H62eK&Reserve3=.
A. Taladriz, A. Healy, E. J. F. Perez, V. H. Garcha, C. R. Marthnez, A. A. M. Alkhaldi, A. A. Eze, M. Kaiser, H. P. de Koning, A. Chana, C. Dardonville, J. Med. Chem., 2012, 55, 2606.
L. T. Archipova, M. M. Archipova, L. E. Bakeeva, A. Zh. Fursova, E. N. Grigorian, A. Yu. Grishanova, E. N. Iomdina, Zh. N. Ivashchenko, L. A. Katargina, O. V. Kilina, N. G. Kolosova, E. P. Kopenkin, N. A. Kovaleva, V. V. Neroev, Y. P. Novikova, P. P. Philippov, O. V. Rubtsova, V. B. Saprunova, I. I. Senin, M. V. Skulachev, L. F. Sotnikova, N. K. Tikhomirova, N. A. Trofimova, I. P. Khoroshilova-Maslova, I. V. Tsapenko, A. I. Shchipanova, V. P. Skulachev, Biochem. (Moscow), 2008, 73, 1317 [Biokhim. (Moscow), 2008, 73, 1641].
E. V. Yani, L. A. Katargina, N. B. Chesnokova, O. V. Beznos, A. Yu. Savchenko, V. A. Vygodin, E. Yu. Gudkova, A. A. Zamyatnin, M. V. Skulachev, Prakt. med. [Pract. Med.], 2012, 1, No. 59, 134 (in Russian).
M. V. Pugachev, N. V. Shtyrlin, E. V. Nikitina, L. P. Sysoeva, T. I. Abdullin, A. G. Iksanova, A. A. Ilaeva, E. A. Berdnikov, R. Z. Musin, Yu. G. Shtyrlin, Bioorg. Med. Chem., 2013, 21, 4388.
M. V. Pugachev, N. V. Shtyrlin, S. V. Sapognikov, L. P. Sysoeva, A. G. Iksanova, E. V. Nikitina, R. Z. Musin, O. A. Lodochnikova, E. A. Berdnikov, Yu. G. Shtyrlin, Bioorg. Med. Chem., 2013, 21, 7330.
N. V. Shtyrlin, A. D. Strel´nik, L. P. Sysoeva, O. A. Lodochnikova, E. N. Klimovitskii, Yu. G. Shtyrlin, Russ. J. Org. Chem. (Engl. Transl.), 2009, 45, 1266 [Zh. Org. Khim., 2009, 45, 1274].
N. V. Shtyrlin, O. A. Lodochnikova, M. V. Pugachev, T. I. Madzhidov, L. P. Sysoeva, I. A. Litvinov, E. N. Klimovitskii, Yu. G. Shtyrlin, Russ. J. Org. Chem. (Engl. Transl.), 2010, 46, 561[Zh. Org. Khim., 2010, 46, 569].
N. V. Shtyrlin, R. S. Pavelyev, M. V. Pugachev, L. P. Sysoeva, R. Z. Musin, Yu. G. Shtyrlin, Tetrahedron Lett., 2012, 53, 3967.
A. U. Ziganshin, O. S. Kalinina, A. D. Strelnik, M. R. Garipov, S. A. Koshkin, L. E. Ziganshina, Yu. G. Shtyrlin, Int. J. Pharmacol., 2015, 11, 400.
R. Serwa, T.-G. Nam, L. Valgimigli, S. Culbertson, C. L. Rector, B.-S. Jeong, D. A. Pratt, N. A. Porter, Chem. Eur. J., 2010, 47, 14106.
K. Dong-Guk, K. Youra, L. Hyunji, L. E. Kyung, N. Tae-Gyu, K. Jung-Ae, J. Byeong-Seon, Eur. J. Med. Chem., 2014, 78, 126.
A. Weisberger, Organic Solveents. Physical Properties and Methods of Purification, International Publishers, New York, 1955, 372 pp.
Y. Murakami, J. Sunamoto, H. Sadamori, H. Kondo, M. Takagi, Bull. Chem. Soc. Jpn, 1970, 43, 2518.
R. Kuhn, H. R. Hensel, Chem. Ber., 1953, 86, 1333.
T. L. Gilkrist, G. M. Iskander, A. K. Yagoub, J. Chem. Soc., Chem. Commun., 1981, 696.
R. F. Lonq, A. L. Morrison, J. Chem. Soc., 1954, 3854.
R. G. Taborsky, J. Org. Chem., 1960, 26, 596.
E. Wilson, M. Tishler, J. Am. Chem. Soc., 1951, 73, 3635.
A. N. Mironov, Rukovodstvo po provedeniyu doklinicheskikh issledovanii lekarstvennykh sredstv [Manual on Conducting Preclinical Studies of Medicines], P. 1, Grif i K, Moscow, 2012, 944 pp. (in Russian).
D. Maron, B. Ames, Mutation Res., 1983, 113, 173.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences N. S. Zefirov on the occasion of his 80th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 2, pp. 0537—0545, February, 2016.
Rights and permissions
About this article
Cite this article
Shtyrlin, N.V., Vafina, R.M., Pugachev, M.V. et al. Synthesis and biological activity of quaternary phosphonium salts based on 3-hydroxypyridine and 4-deoxypyridoxine. Russ Chem Bull 65, 537–545 (2016). https://doi.org/10.1007/s11172-016-1334-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-016-1334-y