Abstract
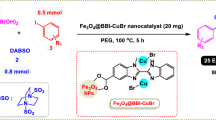
C–H arylation of heterocyclic compounds is a very interesting and important research field in chemistry for the formation of C–C bonds, but unfortunately, few methods have been reported for this purpose. In this attractive and highly efficient methodology, we wish to report that copper (I) chloride immobilized on magnetic nanoparticles modified with benzothiazole-pyrimidine Ligand (Fe3O4@BTH-Pyr-CuCl) is a novel and efficient magnetically recoverable catalyst for carbon–carbon bond formation through C–H arylation of a category of heterocyclic compounds by aryl iodides in the presence of KOAc in PEG under mild conditions. Under this catalytic system, A category of heterocyclic substrates including benzo[d]oxazole, benzo[d]thiazole, 1-methyl-1H-benzo[d]imidazole, 2,5-diphenyloxazole, benzofuran, 2-phenyl-1,3,4-oxadiazole and 2-phenylimidazo[1,2-a]pyridine were used as substrate and the desired products were prepared with high to excellent yields. Reusability tests revealed that the Fe3O4@BTH-Pyr-CuCl catalyst can be reused at least 7 runs without significant decrease in its catalytic efficiency. ICP-OES, VSM and SEM techniques confirmed the recovered Fe3O4@BTH-Pyr-CuCl catalyst was very stable and had high magnetic property despite the utilization for 7 runs.
Graphical Abstract














Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
A.K. Sharma, H. Joshi, A.K. Singh, RSC Adv. 10, 6452 (2020)
Y. Zhang, N. Song, Biol. Mol. Chem. 1, 53 (2023)
V.G. Pandya, S.B. Mhaske, Org. Lett. 16, 3836 (2014)
R. Chawla, L.D.S. Yadav, Org. Biomol. Chem. 17, 4761 (2019)
S. Gupta, J. Synth. Chem. 1, 16 (2022)
M. Kazemi, Nanomater. Chem. 1, 1 (2023)
S. Vajar, M. Mokhtary, Polycycl. Aromat. Compd. 39, 111 (2019)
R. Deilam, F. Moeinpour, F.S. Mohseni-Shahri, Monatshefte für Chem. Chem. Mon. 151, 1153 (2020)
M. Ghobadi, J. Synth. Chem. 1, 84 (2022)
M. Ghobadi, M. Kargar Razi, R. Javahershenas, M. Kazemi, Synth. Commun. 51, 647 (2021)
P. Ghamari Kargar, C. Len, R. Luque, Sustain. Chem. Pharm. 27, 100672 (2022)
R. Arundhathi, D. Damodara, P.R. Likhar, M.L. Kantam, P. Saravanan, T. Magdaleno, S.H. Kwon, Adv. Synth. Catal. 353, 1591 (2011)
L.S. Ardakani, A. Arabmarkadeh, M. Kazemi, Synth. Commun. 51, 856 (2021)
F.M. Moghaddam, M. Eslami, Appl. Organomet. Chem. 32, e4463 (2018)
M.R. Abdi, Biol. Mol. Chem. 1, 1 (2023)
A.R. Sardarian, F. Mohammadi, M. Esmaeilpour, Res. Chem. Intermed. 45, 1437 (2019)
R. Eisavi, A. Karimi, RSC Adv. 9, 29873 (2019)
M. Kazemi, Synth. Commun. 50, 1899 (2020)
A. Noory Fajer, H. Khabt Aboud, H. A. Al-Bahrani, M. Kazemi, Polycycl. Aromat. Compd. 2023, 1–47.
M. Lakshman, J. Synth. Chem. 1, 48 (2022)
S. Sajjadifar, M.A. Zolfigol, F. Tami, J. Chin. Chem. Soc. 66, 307 (2019)
C.–H. Ma, M. Chen, Z.-W. Feng, Y. Zhang, J. Wang, Y.-Q. Jiang, B. Yu, New J. Chem. 45, 9302 (2021)
M. Kamalzare, M.R. Ahghari, M. Bayat, A. Maleki, Sci. Rep. 11, 20021 (2021)
S. Karami, M.G. Dekamin, E. Valiey, P. Shakib, New J. Chem. 44, 13952 (2020)
N. Deibl, K. Ament, R. Kempe, J. Am. Chem. Soc. 137, 12804 (2015)
H. Laatsch, B. Renneberg, U. Hanefeld, M. Kellner, H. Pudleiner, G. Hamprecht, H.-P. Kraemer, H. Anke, Chem. Pharm. Bull. 43, 537 (1995)
T. Zarganes-Tzitzikas, P. Patil, K. Khoury, E. Herdtweck, A. Dömling, Eur. J. Org. Chem. 2015, 51 (2015)
A. Elhampour, F. Nemati, Org. Prep. Proced. Int. 50, 493 (2018)
M.M. Khakzad Siuki, M. Bakavoli, H. Eshghi, Appl. Organomet. Chem. 33, e4774 (2019)
Y. Chen, Z.-J. Zhuo, D.-M. Cui, C. Zhang, J. Organomet. Chem. 749, 215 (2014)
C.-J. Li, Can. J. Chem. 100, 98 (2022)
N.G. Schmidt, E. Eger, W. Kroutil, ACS Catal. 6, 4286 (2016)
H. Zhong, B. Morandi, Nat. Synth. 1, 264 (2022)
L.E. Zetzsche, A.R.H. Narayan, Nat. Rev. Chem. 4, 334 (2020)
V. Resch, J.H. Schrittwieser, E. Siirola, W. Kroutil, Curr. Opin. Biotechnol. 22, 793 (2011)
J. Kim, S.H. Hong, ACS Catal. 7, 3336 (2017)
D.R. Heitz, J.C. Tellis, G.A. Molander, J. Am. Chem. Soc. 138, 12715 (2016)
H. Bohra, M. Wang, J. Mater. Chem. A 5, 11550 (2017)
I.B. Seiple, S. Su, R.A. Rodriguez, R. Gianatassio, Y. Fujiwara, A.L. Sobel, P.S. Baran, J. Am. Chem. Soc. 132, 13194 (2010)
J. Ren, C. Pi, X. Cui, Y. Wu, Green Chem. 24, 3017 (2022)
S. Haldar, S. Mandal, C.K. Jana, Handbook of CH-Functionalization (Wiley, Hoboken, 2022), p.1
C.A. Obasanjo, A.S. Zeraati, H.S. Shiran, T.N. Nguyen, S.M. Sadaf, M.G. Kibria, C.-T. Dinh, J. Mater. Chem. A 10, 20059–20070 (2022)
L. Chen, A. Noory Fajer, Z. Yessimbekov, M. Kazemi, M. Mohammadi, J. Sulfur Chem. 40, 451 (2019)
K. Khazenipour, F. Moeinpour, F.S. Mohseni-Shahri, J. Chin. Chem. Soc. 68, 121 (2021)
Y. Fang, S. Chen, L.-Y. Chang, RSC Adv. 14, 812 (2024)
M.M. Khodaei, A. Alizadeh, M. Haghipour, Res. Chem. Intermed. 45, 2727 (2019)
G. Huang, H. Sun, X. Qiu, C. Jin, C. Lin, Y. Shen, J. Jiang, L. Wang, Org. Lett. 13, 5224 (2011)
N.-N. Jia, X.-C. Tian, X.-X. Qu, X.-X. Chen, Y.-N. Cao, Y.-X. Yao, F. Gao, X.-L. Zhou, Sci. Rep. 7, 43758 (2017)
P.H. Tran, A.-H. Thi Hang, RSC Adv. 8, 11127 (2018)
R.G. Kalkhambkar, K.K. Laali, Tetrahedron Lett. 53, 4212 (2012)
H.T. Nguyen, T.H. Nguyen, D.D. Pham, C.T. Nguyen, P.H. Tran, Heliyon 7, e08309 (2021)
O. Yuen, C. So, W. Wong, F. Kwong, Synlett 23, 2714 (2012)
Q. Tian, W. Luo, Z. Gan, D. Li, Z. Dai, H. Wang, X. Wang, J. Yuan, Molecules 24, 174 (2019)
O.V. Khazipov, K.E. Shepelenko, S.B. Soliev, K.A. Nikolaeva, V.M. Chernyshev, V.P. Ananikov, ChemCatChem 14, e202201055 (2022)
G. Chakraborty, R. Mondal, A.K. Guin, N.D. Paul, Org. Biomol. Chem. 19, 7217 (2021)
J. Zhang, X. Zhao, P. Liu, P. Sun, J. Org. Chem. 84, 12596 (2019)
A. Pal, A. Thakur, Org. Biomol. Chem. 20, 8977 (2022)
Y. Li, J. Jin, W. Qian, W. Bao, Org. Biomol. Chem. 8, 326 (2010)
K.M. Liu, L.Y. Liao, X.F. Duan, Chem. Commun. 51, 1124 (2015)
A. Moazzam, S.M. Farid, N. Khaleghi, N. Alizadeh, M. Mahdavi, New J. Chem. 46, 10814 (2022)
M.-A. Hiebel, Y. Fall, M.-C. Scherrmann, S. Berteina-Raboin, Eur. J. Org. Chem. 2014, 4643 (2014)
S. Wang, K. Wang, X. Kong, S. Zhang, G. Jiang, F. Ji, Adv. Synth. Catal. 361, 3986 (2019)
Acknowledgements
This work was supported by the General Project of Liaoning Provincial Department of Education (CN) [LJKMZ20221682] and [2023J0917], the Doctoral Initiation Foundation of Liaoning Institute of Science and Tenchnology [2307B22], LiaoNing Basic Applied Research Program (Youth Program), [2023JH2/101600066].
Author information
Authors and Affiliations
Contributions
Jingming Zhao:Performing experimental works. Xudong Luo: Performing experimental works. Xiaoliang Li:Performing experimental works and taking analyzes. Li-Yuan Chang: Manager project.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, J., Luo, X., Li, X. et al. Construction of Fe3O4@BTH-Pyr-CuCl nanocomposite as a highly active magnetically reusable catalyst for arylation of a category of heterocycles. Res Chem Intermed 50, 2131–2156 (2024). https://doi.org/10.1007/s11164-024-05250-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-024-05250-4