Abstract
In this study, SBA-15 was synthesized and functionalized with [3-(N,N-dimethylamino)-propyl] trimethoxysilane (AF-SBA-15) using a post-grafting method and evaluated for use in the removal of an anionic synthetic dye (Congo Red, CR) from aqueous media. The synthesized mesoporous materials were characterized by small-angle XRD, N2 adsorption–desorption, FT-IR, and SEM techniques. The ordered hexagonal structure of pure SBA-15 and amine-functionalized SBA-15 (AF-SBA-15) was confirmed by small-angle XRD analysis; surface area, pore size, and pore diameter were determined by nitrogen adsorption–desorption analysis. Kinetics showed that the process reached equilibrium after 90 min; adsorption followed pseudo-second-order kinetic model. Maximum efficiency was obtained at the unadjusted pH (8.8) of the aqueous media, and the efficiency was negatively affected by CR concentration, but increased with dose and temperature. While pure SBA-15 could not adsorb CR, the highest adsorption capacity and efficiency obtained with AF-SBA-15 were calculated to be 186.41 mg/g and 95.3%, respectively. The data were successfully fitted to Langmuir adsorption isotherm (R2 > 0.99), and consistently, thermodynamics signified possible physico-chemical interactions between CR and AF-SBA-15. The process was shown to be endothermic and non-spontaneous. The results show that amine-functionalized SBA-15 can be used to efficiently remove toxic anionic colorants from aqueous media.
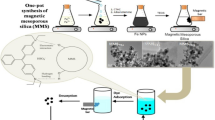
Graphical abstract
The proposed mechanisms for the adsorption of CR onto AF-SBA-15 under acidic and neutral conditions of media.











Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this manuscript.
Abbreviations
- AE:
-
Adsorption efficiency, (%)
- AF-SBA-15:
-
Amine-functionalized mesoporous silica
- B:
-
Temkin equilibrium binding constant, (J/mol)
- BET:
-
Brunauer–Emmett–Teller method
- BJH:
-
Barrett–Joyner–Halenda method
- C o :
-
Initial concentration of CR, (mg/L)
- C e :
-
Equilibrium concentration of CR, (mg/L)
- CR:
-
Congo Red
- D BJH :
-
Pore diameter according to BJH method, (nm)
- I :
-
Boundary layer diffusion effects, (mg/g)
- ID:
-
Intraparticle diffusion
- k 1 :
-
Rate constant of PFO kinetic model, (1/min)
- k 2 :
-
Rate constant of PSO kinetic model, (g/mg min)
- k id :
-
Rate constant of ID model, (mg/g1 min0.5)
- K F :
-
Adsorption capacity of Freundlich isotherm model, (L/mg)
- K L :
-
Langmuir equilibrium constant, (L/mg)
- K T :
-
Temkin constant related to the adsorption heat, (L/mg)
- m :
-
Mass of AF-SBA-15, (g)
- n :
-
Freundlich adsorption intensity
- p/p o :
-
Relative pressure
- PFO:
-
Pseudo-first-order
- pHpzc :
-
PH at point of zero charge
- PSO:
-
Pseudo-second-order
- q e :
-
Amount of CR adsorbed per gram adsorbent at equilibrium, (mg/g)
- q t :
-
Amount of CR adsorbed per gram adsorbent at time t, (mg/g)
- q max :
-
Maximum amount of CR adsorbed per gram adsorbent, (mg/g)
- R :
-
Gas constant, (8.314 J/mol K)
- R L :
-
Dimensionless separation factor
- R 2 :
-
Determination coefficient
- SBA-15:
-
Mesoporous silica
- S BET :
-
Surface area according to BET method, (m2/g)
- t :
-
Time, (min)
- T :
-
Temperature, (K or °C)
- V :
-
Volume of the aqueous solution, (L)
- V BJH :
-
Pore volume according to BJH, (cc/g)
- XRD:
-
X-ray diffraction
- α :
-
Elovich initial adsorption rate, (mg/g min)
- β :
-
Elovich desorption constant, (g/mg)
- [ ]:
-
Concentration of the solute, (mol/L)
- ΔG°:
-
Change in Gibbs free energy, (kJ/mol)
- ΔH°:
-
Change in enthalpy, (kJ/mol)
- ΔS°:
-
Change in entropy, (J/mol K)
References
S. Dawood, T.K. Sen, J. Chem. Proc. Eng. 1, 1 (2014)
D.D. Asouhidou, K.S. Triantafyllidis, N.K. Lazaridis, K.A. Matis, S. Kim, T.J. Pinnavaia, Microporous Mesoporous Mater. 117, 257 (2009)
W. Huanga, J. Xua, D. Lua, J. Denga, G. Shic, T. Zhoua, Appl. Surf. Sci. 462, 453 (2018)
R.V. Kandisa, K.B. Shaik, R. Gopinath, J. Bioremed. Biodegrad. 7, 1 (2016)
Z. Salahshoor, A. Shahbazi, Eur. J. Environ. Sci. 4, 116 (2014)
S.M. Hafezian, P. Biparva, A. Bekhradnia, S.N. Azizi, Adv. Powder Technol. 32, 779 (2021)
P.N.E. Diagboya, E.D. Dikio, Microporous Mesoporous Mater. 266, 252 (2018)
J.S. Beck, J.C. Vartuli, W.J. Roth, M.E. Leonowicz, C.T. Kresge, K.D. Schmitt, C.T.-W. Chu, D.H. Olson, E.W. Sheppard, S.B. McCullen, J.B. Higgins, J. Schlenker, J. Am. Chem. Soc. 114, 10834 (1992)
Z. Wu, D. Zhao, Chem. Commun. 47, 3332 (2011)
K. Northcott, H. Kokusen, Y. Komatsu, G. Stevens, Sep. Sci. Technol. 41, 1829 (2006)
Y. Wei, D. Jin, T. Ding, W.H. Shih, X. Liu, S.Z. Cheng, Q. Fu, Adv. Mater. 10, 313 (1998)
J.M. Kim, G.D. Stucky, Chem. Commun. 13, 1159 (2000)
S.H. Wu, C.Y. Mou, H.P. Lin, Chem. Soc. Rev. 42, 3862 (2013)
W. Li, J. Liu, D. Zhao, Nat. Rev. Mater. 1, 1 (2016)
D. Zhao, J. Feng, Q. Huo, N. Melosh, G.H. Fredrickson, B.F. Chmelka, G.D. Stucky, Science 279, 548 (1998)
L. Hajiaghababaei, S. Abozari, A. Badiei, P. Zarabadi Poor, S.D. Abkenar, M.R. Ganjali, G.M. Ziarani, Iran. J. Chem. Chem. Eng. 36, 97 (2017)
A. Larki, S.J. Saghanezhad, M. Ghomi, Microchem. J. 169, 106601 (2021)
H. Wang, C.J. Liu, Appl. Catal. B: Environ. 106, 672 (2011)
X.K. Li, W.J. Ji, J. Zhao, S.J. Wang, C.T. Au, J. Catal. 236, 181 (2005)
T.H. Liou, M.H. Lin, Sep. Sci. Technol. 55, 431 (2019)
Y. Shen, N. Jiang, S. Liu, C. Zheng, X. Wang, T. Huang, Y. Guo, R. Bai, J. Environ. Chem. Eng. 6, 5420 (2018)
K. Dashtian, R. Zare-Dorabei, R. Jafarinia, M.S. Tehrani, J. Environ. Chem. Eng. 5, 5233 (2017)
X. Wang, Z. Zhang, Y. Zhao, K. Xia, Y. Guo, Z. Qu, R. Bai, Nanomaterials 8, 673 (2018)
Z. Zhang, K. Xia, Z. Pan, C. Yang, X. Wang, G. Zhang, Y. Guo, R. Bai, Appl. Surf. Sci. 500, 143970 (2020)
J. Shen, S. Zhang, Z. Zeng, J. Huang, Y. Shen, Y. Guo, ACS Omega 6, 25791 (2021)
L. Dolatyari, M. Shateri, M.R. Yaftian, S. Rostamnia, Sep. Sci. Technol. 54, 2863 (2019)
N. Yaghobi, L. Hajiaghababaei, A. Badiei, M.R. Ganjali, G.Z. Mohammadi, J. Chem. Health Risks 9, 253 (2019)
Y. Zhang, X. Cao, J. Sun, G. Wu, J. Wang, D. Zhang, J. Sol–Gel Sci. Technol. 94, 658 (2020)
X. Wang, G. Ji, G. Zhu, C. Song, H. Zhang, C. Gao, Sep. Purif. Technol. 209, 623 (2019)
X. Zheng, Z. Song, E. Liu, Y. Zhang, Z. Li, J. Chem. Eng. Data 65, 746 (2020)
Y. Ding, Q. Xian, E. Wang, X. He, Z. Jiang, H. Dan, W. Zhu, New J. Chem. 44, 13707 (2020)
H. Chaudhuri, S. Dash, A. Sarkar, Ind. Eng. Chem. Res. 55, 10084 (2016)
Z. Abid, A. Hakiki, B. Boukoussa, F. Launay, H. Hamaizi, A. Bengueddach, R. Hamacha, J. Mater. Sci. 54, 7679 (2019)
M. Mirzaie, A. Rashidi, H.A. Tayebi, M.E. Yazdanshenas, J. Chem. Eng. Data 62, 1365 (2017)
M. Mirzaie, A. Rashidi, H.A. Tayebi, M.E. Yazdanshenas, J. Chem. Health Risks 7, 285 (2017)
F. Bahalkeh, R.Z. Mehrabian, M. Ebadi, Int. J. Biol. Macromol. 164, 818 (2020)
A.J. Khan, J. Song, K. Ahmed, A. Rahim, P.L.O. Volpe, F. Rehman, J. Mol. Liq. 298, 111957 (2020)
S. Masoudnia, M.H. Juybari, R.Z. Mehrabian, M. Ebadi, F. Kaveh, Int. J. Biol. Macromol. 165, 118 (2020)
B. Boukoussa, A. Hakiki, S. Moulai, K. Chikh, D.E. Kherroub, L. Bouhadjar, D. Guedal, K. Messaoudi, F. Mokhtar, R. Hamacha, J. Mater. Sci. 53, 7372 (2018)
B. Boukoussa, A. Mokhtar, A. El Guerdaoui, M. Hachemaoui, H. Ouachtak, S. Abdelkrim, A.A. Addi, S. Babou, B. Boudina, A. Bengueddach, R. Hamacha, J. Mol. Liq. 333, 115976 (2021)
M. Abboud, T. Sahlabji, M.A. Haija, A.A. El-Zahhar, S. Bondock, I. Ismail, S.M.A.S. Keshk, New J. Chem. 44, 2291 (2020)
P. Sun, L. Xu, X. Jiang, H. Zhang, W. Zhu, Ind. Eng. Chem. Res. 58, 2945 (2019)
D. Zhao, Q. Huo, J. Feng, B.F. Chmelka, G.D. Stucky, J. Am. Chem. Soc. 120, 6024 (1998)
C. Hess, J.D. Hoefelmeyer, T.D. Tilley, J. Phys. Chem. B 108, 9703 (2004)
L.S. Balistrieri, J.W. Murray, Am. J. Sci. 281, 788 (1981)
H. Chaudhuri, S. Dash, A. Sarkar, New J. Chem. 40, 3622 (2016)
R. Lu, W. Gan, B.H. Wu, Z. Zhang, Y. Guo, H.F. Wang, J. Phys. Chem. B 109, 14118 (2005)
T. Tang, Y. Zhao, Y. Xu, D. Wu, J. Xu, F. Deng, Appl. Surf. Sci. 257, 6004 (2011)
M. Mohammadi, A.J. Hassani, A.R. Mohamed, G.D. Najafpour, J. Chem. Eng. Data 55, 5777 (2010)
F.A. Pavan, S.L.P. Dias, E.C. Lima, E.V. Benvenutti, Dyes Pigm. 76, 64 (2008)
K.M. Park, I.L. Yoon, S.S. Lee, G. Choi, J.S. Lee, Dyes Pigm. 54, 155 (2002)
K. Diouri, A. Kherbeche, A. Chaqroune, Int. J. Innov. Res. Sci. Eng. Technol. 4, 267 (2015)
S. Banerjee, M.C. Chattopadhyaya, Arab. J. Chem. 10, 1629 (2017)
S.Y. Lagergren, Kungl. Svens. Vetenskapsakad. 24, 1 (1898)
Y.S. Ho, G. McKay, Process Biochem. 34, 451 (1999)
S.Y. Elovich, O.G. Larinov, Izv. Akad. Nauk. SSSR, Otd. Khim. Nauk, 2, 209 (1962)
W.J. Weber, JC Morris in: Proc. Int. Conf. Water Pollution Symposium, 2, 231 (1962)
D. Kavitha, C. Namasivayam, Bioresour. Technol. 98, 14 (2007)
J. Liu, F. Chen, C. Li, L. Lu, C. Hu, Y. Wei, P. Raymer, Q. Huang, J. Clean. Prod. 208, 552 (2019)
K. Ali, H. Zeidan, M.E. Marti, Desalin. Water Treat. 227, 412 (2021)
H. Zeidan, M.E. Marti, J. Chem. Eng. Data 64, 2718 (2019)
A. Khaled, A. El Nemr, A. El-Sikaily, O. Abdelwahab, Desalination 238, 210 (2009)
R.S.D. Farias, H.L.D.B. Buarque, M.R.D. Cruz, L.M.F. Cardoso, T.D.A. Gondim, V.R.D. Paulo, Eng. Sanit. e Ambient. 23, 1053 (2018)
F. Sholehah, P. Taba, Y. Hala, AIP Conf. Proc. 2360, 050013 (2020)
E. Abdelkader, L. Nadjia, V. Rose-Noëlle, Int. J. Ind. Chem. 7, 53 (2016)
T. Şişmanoğlu, G.S. Pozan, Desalin. Water Treat. 57, 13318 (2016)
M. Ghaedi, H. Tavallali, M. Sharifi, S.N. Kokhdan, A. Asghari, Spectrochim. Acta, Part A 86, 107 (2012)
I.D. Mall, V.C. Srivastava, N.K. Agarwal, I.M. Mishra, Chemosphere 61, 492 (2005)
A. Tor, Y. Cengeloglu, J. Hazard. Mater. 138, 409 (2006)
C. Xia, Y. Jing, Y. Jia, D. Yue, J. Ma, X. Yin, Desalination 265, 81 (2011)
H. Zeidan, D. Ozdemir, N. Kose, E. Pehlivan, G. Ahmetli, M.E. Marti, Desalin. Water Treat. 202, 283 (2020)
I. Langmuir, J. Am. Chem. Soc. 38, 2221 (1916)
P. Velmurugan, J. Shim, B.T. Oh, Res. Chem. Intermed. 42, 5937 (2016)
H. Freundlich, Z. Phys. Chem. 57, 385 (1907)
Y. Zheng, Y. Liu, A. Wang, Ind. Eng. Chem. Res. 51, 10079 (2012)
M.I. Temkin, V. Pyzhev, Acta Phys. Chim. USSR 12, 327 (1940)
H. Zhang, Y. Ruan, Y. Feng, M. Su, Z. Diao, D. Chen, L. Hou, P. Lee, K. Shih, L. Kong, J. Colloid Interface Sci. 536, 180 (2019)
H.A. Tabti, B. Medjahed, M. Boudinar, A. Kadeche, N. Bouchikhi, A. Ramdani, S. Taleb, M. Adjdir, Res. Chem. Intermed. 48, 2683 (2022)
S.T. Yang, S. Chen, Y. Chang, A. Cao, Y. Liu, H. Wang, J. Colloid Interface Sci. 359, 24 (2011)
Acknowledgements
The authors wish to acknowledge Konya Technical University for the support and facilities used in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeidan, H., Can, M. & Marti, M.E. Synthesis, characterization, and use of an amine-functionalized mesoporous silica SBA-15 for the removal of Congo Red from aqueous media. Res Chem Intermed 49, 221–240 (2023). https://doi.org/10.1007/s11164-022-04876-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04876-6