Abstract
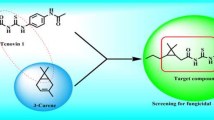
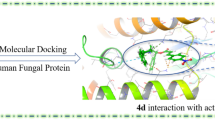
In search of potent antifungal agents derived from natural product 3-carene, 19 novel (Z)-3-caren-5-one oxime ethers 3a–3s were synthesized by multi-step reaction and characterized by UV–Vis, IR, NMR, ESI–MS and elemental analysis. The in vitro antifungal activity of all the target compounds was preliminarily evaluated, and the test results indicated that some of the target compounds displayed excellent inhibitory activity against R. Solani. For example, the inhibition percentages against R. Solani of compounds 3b (R = 2'–CH3), 3c (R = 3'–CH3) and 3i (R = 3'–Cl) were 98.4, 93.7 and 94.9%, respectively, which were superior or comparable to that of positive control chlorothalonil. The computational simulation study was also carried out to elucidate the structure–activity relationship (SAR) of the title compounds. Firstly, an effective and reasonable 3D-QSAR model, valuable for the optimization of the title compounds, was built by CoMFA method. In addition, DFT calculation uncovered that both 3-carene moiety and oxime ether group were the potential pharmacophores of compound 3b with the best antifungal activity. Meanwhile, molecular docking and dynamic simulation were employed for investigating the binding mode and dynamic stability of compound 3b in the Q0-site of cytochrome bc1 complex, using original ligand JZV (trifloxystrobin) as comparison. Compounds 3b (R = 2'–CH3) deserved further study as a leading compound.







Similar content being viewed by others
References
M. Karapandzova, G. Stefkov, I.C. Karanfilova, T.K. Panovska, J.P. Stanoeva, M. Stefova, S. Kulevanova Rec. Nat. Prod. 13, 50 (2019)
J.Q. Cao, S.S. Guo, Y. Wang, X. Pang, Z.F. Geng, S.S. Du Ecotoxicol, Environ. Saf. 160, 342 (2018)
L.Z. He, J. Wang, Z.D. Zhao, Y.X. Chen, Y. Gu Chem. Ind. Forest Prod. 31, 122 (2011)
J.D. Langsi, E.N. Nukenine, K.M. Oumarou, H. Moktar, C.N. Fokunang, G.N. Mbata Insects 11, 540 (2020)
H.Z. Shu, W.M. Zhang, Y.H. Yun, W.J. Chen, Q.P. Zhong, Y.Y. Hu, H.M. Chen, W.X. Chen Food Chem. 329, 127220 (2020)
H.Z. Shu, H.M. Chen, X.L. Wang, Y.Y. Hu, Y.H. Yun, Q.P. Zhong, W.J. Chen, W.X. Chen Molecules 24, 3246 (2019)
J. Woo, H. Yang, M. Yoon, C.G. Gadhe, A.N. Pae, S. Cho, C.J. Lee Exp. Neurobiol. 28, 593 (2019)
R.G. Kelsey, D.J. Westlind Agric. For. Entomol. 23, 243 (2020)
R.G. Kelsey, D.J. Westlind Agric. For. Entomol. 20, 272 (2018)
F. Burcul, I. Blazevic, M. Radan, O. Politeo Curr. Med. Chem. 27, 4297 (2020)
G.Q. Kang, W.G. Duan, G.S. Lin, Y.P. Yu, X.Y. Wang, S.Z. Lu Molecules 24, 477 (2019)
M. Huang, W.G. Duan, G.S. Lin, K. Li, Q. Hu Molecules 22, 1538 (2017)
Q.A. Zhang, G.S. Lin, W.G. Duan, S.Y. Zhao, J.M. He, F.H. Lei ChemistrySelect 6, 4515 (2021)
Q.D. Jin, F.B. Li, Y. Li, F. Jin, L. Jiang Res. Chem. Intermed. 41, 7695 (2015)
M.L. Wang, Y. Du, C. Ling, Z.K. Yang, B.B. Jiang, H.X. Duan, J. An, X.H. Li, X.L. Yang Pest Manage. Sci. 77, 3910 (2021)
R.J. Xia, T. Guo, M. Chen, S.J. Su, J. He, X. Tang, S.C. Jiang, W. Xue New J. Chem. 43, 16461 (2019)
S.J. Su, M. Chen, Q. Li, Y.H. Wang, S. Chen, N. Sun, C.W. Xie, Z.Y. Huai, Y.J. Huang, W. Xue Bioorg. Med. Chem. 32, 115999 (2021)
N. Gupta, A. Qayum, A. Raina, R. Shankar, S. Gairola, S. Singh, P.L. Sangwan Eur. J. Med. Chem. 145, 511 (2018)
P. Lei, X.J. Ding, Y.Y. Wu, Z.Q. Ma Chin. J. Org. Chem. 39, 2070 (2019)
B.L. Trumpower J. Biol. Chem. 265, 11409 (1990)
A.R. Crofts Annu. Rev. Physiol. 66, 689 (2004)
E.A. Berry, M. Guergova-Kuras, L.S. Huang, A.R. Crofts Annu. Rev. Biochem. 69, 1005 (2000)
N. Fisher, B. Meunier, G.A. Biagini FEBS Lett. 594, 2935 (2020)
H. Cheng, Y. Fu, Q. Chang, N. Zhang, M.W. Bu, Y. Niu, Q.Y. Wu, C. Chen, F. Verpoort Chin. Chem. Lett. 29, 1897 (2018)
C. Chen, Q.Y. Wu, L.Y. Shan, B. Zhang, F. Verpoort, G.F. Yang RSC Adv. 6, 97580 (2016)
X.L. Zhu, R. Zhang, Q.Y. Wu, Y.J. Song, Y.X. Wang, J.F. Yang, G.F. Yang J. Agric. Food Chem. 67, 2774 (2019)
L. Musso, A. Fabbrini, S. Dallavalle Molecules 25, 4582 (2020)
FRAC classification of fungicides. Accessed Oct. 25, 2021.
N.Y. Chen, W.G. Duan, G.S. Lin, L.Z. Liu, R. Zhang, D.P. Li Mol. Divers. 20, 897 (2016)
X. Wang, W.G. Duan, G.S. Lin, M. Chen, F.H. Lei Res. Chem. Intermed. 47, 4029 (2021)
B.Y. Li, G.S. Lin, W.G. Duan, X.Y. Wang, B. Cen J. Agric. Food Chem. 69, 12956 (2021)
N.N. Su, Y. Li, S.J. Yu, X. Zhang, X.H. Liu, W.G. Zhao Res. Chem. Intermed. 39, 759 (2013)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, G.A. Petersson, H. Nakatsuji, M.C. X. Li, A. Marenich, J. Bloino, B.G. Janesko, R. Gomperts, B. Mennucci, H.P. Hratchian, J.V. Ortiz, A.F. Izmaylov, J.L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V.G. Zakrzewski, N.R. J. Gao, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J.A. Montgomery, J.E.P. Jr., F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S.I.J. Tomasi, M. Cossi, J.M. Millam, M. Klene, C. Adamo, R. Cammi, J.W. Ochterski, R.L. Martin, K. Morokuma, O. Farkas, J.B. Foresman, D.J. Fox, (Gaussian, Inc., Wallingford CT, 2016)
R.A. Laskowski, M.B. Swindells J. Chem. Inf. Model. 51, 2778 (2011)
D. van Der Spoel, E. Lindahl, B. Hess, G. Groenhof, A.E. Mark, H.J. Berendsen J. Comput. Chem. 26, 1701 (2005)
W.F. van Gunsteren, S.R. Billeter, A.A. Eising, P.H. Hunenberger, P. Krüger, A.E. Mark, W.R.P. Scott, I.G. Tironi, Biomolecular simulation: the GROMOS96 manual, user guide, (vdf Hochschulverlag AG an der ETH Zurich, Zurich, 1996)
J. Lemkul Living J. Comp. Mol. Sci. 1, 5068 (2019)
A.W. Schuttelkopf, D.M. van Aalten Acta Crystallogr. Sect. D. Biol. Crystallogr. 60, 1355 (2004)
Acknowledgements
The authors are grateful to the State Key Laboratory of Element-Organic Chemistry, Nankai University, China, for the bioassay test. Also, the computational simulation section of this work was supported by the high-performance computing platform of Guangxi University.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 31870556).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, BY., Kang, GQ., Huang, M. et al. Synthesis, bioactivity and computational simulation study of novel (Z)-3-caren-5-one oxime ethers as potential antifungal agents. Res Chem Intermed 48, 2135–2153 (2022). https://doi.org/10.1007/s11164-022-04690-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04690-0