Abstract
Recently, magnetic iron oxide nanoparticles functionalized with various organic groups increasing attention in the synthesis of the organic compounds. In this study, ionic liquid nano-magnetic pyridinium-tribromide (MNPs@SiO2-Pr-AP-tribromide) as a new nano-catalyst was synthesized. The structure of the catalyst was identified by FT-IR, TGA, XRD, FE-SEM, EDX, EDS, TEM and VSM analysis. The nano-catalyst was efficient for the synthesis of quinoline derivatives through the one-pot reaction of 2-amino-5-chlorobenzophenone and pentane-2,4-dione. The advantages of this catalyst are easy preparation, large surface area, renewability, high thermal stability, high activity, shortening reaction time, improving efficiency, easy purification, and mild reaction conditions.
Graphical abstract
The reaction between 2-aminoarylketone and carbonyl compound is now considered as a major strategy for synthesis of quinoline derivatives. Herein, we employed nano-magnetic pyridiniunm-tribromide (MNPs@SiO2-Pr-AP-tribromide) as a versatile catalyst for the fabrication of novel derivatives of quinoline.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Green chemistry is a strategy that aims to design processes with the least chemical hazards to the environment in the global community. Accordingly, it seeks to use safe methods to increase efficiency, reduce the use of raw materials and solvents, use renewable and biodegradable materials [1]. ILs ionic liquids consist of salts with melting points below 100 ℃, which are made up of large organic cations or organic/inorganic anions. ILs can be effective in realizing the principles of green chemistry in chemical reactions [2]. Extensive properties of ionic liquids include chemical and thermal stability [3], electrical capability [4], non-flammability and non-volatility [5], low toxicity and low vapor pressure [6], high dissolution strength and designability [7]. Also, due to the wide and varied applications of ionic liquids, it can be used as a suitable reagent, catalyst and solvent [8], batteries and fuel cells [9], ionogels, capacitors, and lubricants [2]. Despite all the advantages of ionic liquids, these materials have disadvantages such as high viscosity, lack of economic efficiency due to high consumption in organic reactions, time-consuming recycling that causes some of them to be wasted, and environmental pollution. To solve the mentioned problems, ionic liquids have been fixed on the surface of magnetic nanoparticles. This method increases the activity and selectivity in organic reactions, reduces the amount of catalyst consumption, and also easy separation by external magnet [10,11,12,13]. Different types of ionic liquid catalysts such as [DSIM][AlCl3]x−@CS [5], Fe3O4@SiO2@Propyl-HMTA [14], IL@Fe3O4 [15], [EMIM][EtSO4] [16], Fe3O4@SiO2@(mim)[FeCl4] [17], Chol-MNPs [18] have been used in organic synthesis reactions.
Bromine compounds are used as valuable intermediates in a wide range of industrial, biological, and chemical processes, and as precursors to the formation of C–C and C–X bonds (X = heteroatoms). On the other hand, the use of bromine liquid for broming has disadvantages such as toxicity, corrosion, and high volatility that have limited its use, so in order to reduce these limitations, chemists with the green chemistry approach designed catalysts that could produce bromine [19, 20]. Recently, the fixation of N-tri-bromide on MNP magnetic nanoparticles as a safe, efficient, and recyclable catalyst has been widely studied in organic synthesis [21].
Quinoline is one of the most important and prominent N-heterocyclic structure that has been considered in a wide range of natural, synthetic, and medicinal compounds [22]. Quinolines have properties such as anti-malarial and anti-SARS-CoV-2 [23], corrosion inhibitor [24], HMG-CoA reductase inhibiting [25], antileishmanial, antitubercular, antitumor [26], antihypertensive [27], antibacterial, anti-asthmatic [28], anti-inflammatory [29], anticancer, antioxidant, OLEDs fabrication [30], antifungal, analgesic [31], anti-HIV [32], anticonvulsant [33], anti-Alzheimer [34], anti-diabetic, and anti-platelet activity [35] (Fig. 1). In this regard, the structural nucleus of quinolines can be constructed in different methods. Friedländer annulation is still one of the most simple, and most attractive methods of synthesis of polysubstituted quinolines. Quinoline synthesis in this method, which involves a condensation followed by a cyclodehydration between 2-aminoarylketones and α-methylene ketones, is catalyzed by both acids and bases [36, 37]. In recent years, metal–organic framework (MOFs) catalysts such as UiO-66(Hf) [38] and Zn-MOF [39], chiral phosphoric acid (CPA) [40], Solid SiO2/H2SO4 [41], ionic liquid ImBuSO3H [42], Brønsted acid and Lewis acid [43], [GrBenzImi]SO3H [33], graphene oxide carbocatalyst [44], Fe3O4@SiO2/ISN/Cu(II) [45], DDBSA@MNP [46], microwave irradiation [47, 48], and sulfated polyborate [49]. Organosilane [50], Mn(CO)5Br [51], and PANEOSF [52] have also been utilized to be effective for this synthesis. We synthesis a safe and portable catalyst from nano-iron capable of carrying bromine. The catalyst had a good reactivity due to the appropriate concentration of bromine and had little degradation during the reaction. Then from ionic liquid-based nano-magnetic (MNPs@SiO2-Pr-AP-tribromide) [53] to synthesize quinoline derivatives 3(a–i), we used a one-pot reaction between 2-amino aryl ketones (1) and carbonyl compounds (2) (Scheme 1).
Experimental section
Chemicals and instruments
All chemicals were purchased from Merck and Fluka companies and used without refining.
Melting points were measured with open capillary tubes in the device BUCHI 510. Infrared (IR) spectroscopy was conducted on a Perkin Elmer GX FT‐IR spectrometer. Field emission scanning electron microscopy (FE-SEM) was recorded using a ZEISS MODEL SIGMA VP. Proton nuclear magnetic resonance (1H MNR) spectra and carbon nuclear magnetic resonance (13C NMR) spectra have been reported in CDCl3 or DMSO‐d6 with ppm chemical displacement. Mass spectrometer was recorded in a spectrometer Shimadzu QP 1100 BX.
General procedure for preparation of nano-catalyst MNPs@SiO2-Pr-AP-tribromide
Preparation of Fe3O4 MNPs
First, FeCl3.6H2O (5.83 g) and FeCl2.4H2O (2.147 g) were dissolved in 100 mL of deionized water and subjected to magnetic stirring at 80 °C for 10 min. Then, 10 mL of ammonia solution (25% aqueous) was added to the reaction mixture for 30 min. Then, to prepare Fe3O4 nanoparticles, the reaction was refluxed for 10 h. Then, the suspended Fe3O4 nanoparticles were separated by an external magnet and washed with distilled water and ethanol, and dried in an oven at 80 °C for 12 h.
Preparation of MNPs coated by silica (Fe3O4@SiO2 MNPs)
1 g of Fe3O4 nanoparticles were added to a mixture of EtOH (20 mL), deionized water (80 mL), 4 mL ammonia (25% aqueous), and dispersed by sonication for 30 min. Then, 2 mL of tetraethyl orthosilicate (TEOS) was added to the reaction mixture and stirred at room temperature for 3 h. Finally, the Fe3O4@SiO2 core–shell nanoparticles were separated using an external magnet and washed with deionized water and ethanol to remove unreacted tetraethyl orthosilicate. The nanoparticles were dried in an oven at 60 °C.
Preparation of Fe3O4@SiO2-Pr-Cl containing silane
In order to prepare Fe3O4@SiO2-Pr-Cl nanoparticles, 1 g of Fe3O4@SiO2 nanoparticles were added to a mixture of 3-chloropropyltriethoxysilane (CPTES) (3 mL) and toluene (3 mL) and dispersed for 30 min. It was then refluxed at 80 °C for 8 h. It was then separated by an external magnet and placed in an oven to dry.
Preparation of Fe3O4@SiO2-Pr-AP
To 100 mL DMF, Fe3O4@SiO2-Pr-Cl (1 g) and 4-aminopyridine (0.94 g) were added and dissolved and dispersed for 20 min and refluxed for 24 h at 80 °C. AP-SCMNPs were separated by an external magnet and obtained by washing with DMF by drying at 60 °C.
Preparation of MNPs@SiO2-Pr-AP-tribromide (ionic liquid)
2 mL HBr (47%) was injected dropwise into AP-SCMNPS (1 g) and stirred at room temperature for 30 min. Finally, the product (1 g) was dispersed in CCl4 (4 mL) for 30 min using ultrasound. Br2 (2 mL) was then injected into the mixture and stirred at room temperature for 4 h. Then, the final product of MNPs@SiO2-Pr-AP-tribromide was separated by an external magnet, washed with water and ethanol, and placed in an oven for drying (Scheme 2).
General procedure for synthesis of 1-(6-chloro-2-methyl-4-phenylquinolin-3-yl) ethen-1-one
A mixture of 2-amino-5-chlorobenzophenone (1 mmol), pentane-2,4-dione (1 mmol), and MNPs @SiO2@-Pr-AP-tribromide (0.1 g) in 3 mL ethanol was refluxed. Reaction progression was assessed by TLC (n-hexane/ethyl acetate, 10:3). After completing the reaction, the reaction mixture was cooled, the catalyst was separated from the reaction mixture by an external magnet and washed with hot ethanol. After solvent evaporation, the crude product was crystallized in ethanol, and the product was obtained with good efficiency.
Results and discussion
To identify the presence of functional groups in the catalyst MNPs@SiO2-Pr-AP-tribromide, Fourier transform infrared (FT-IR) spectroscopy was performed (Fig. 2). The FT-IR spectrum of Fe3O4, Fe3O4@SiO2, Fe3O4@ SiO2-PrCl, Fe3O4@SiO2-Pr-AP, and MNPs@SiO2-Pr-AP-tribromide nanoparticles in the wavenumber range of 4000–400 cm−1 is shown in (Fig. 2).
The first FT-IR spectrum (Fig. 2a) is related to iron oxide nanoparticles, and the index peak in region 574 cm−1 is associated with the stretching vibrations of the bond Fe–O. Also the broad peak of the OH group is clearly observed on the surface of Fe3O4 nanoparticles in the area of 3200–3500 cm−1 [54]. The second FT-IR spectrum (Fig. 2b) is associated with silicon-coated iron oxide nanoparticles (Fe3O4@SiO2). The broad peak in region 3431 cm−1 corresponds to the OH stretching frequency of silanol groups (Si–OH). The index peaks in 958, 804, and 1098 cm−1 are also attributed to symmetric Si–O–Si, symmetric Si–O, and asymmetric Si–O–Si stretching vibrations, respectively [55, 56]. The FT-IR spectra of the nanoparticles Fe3O4@SiO2@Si(CH2)3Cl (Fig. 2c), the weak peak shown at 2932 cm−1 is related to the symmetric stretching vibrations of aliphatic CH2 groups [57]. The peaks appearing at 3400, 3141, and 1627 cm−1 correspond to the stretching frequencies of the OH, NH, and C=N groups of Fe3O4@SiO2-Pr-AP, respectively. Therefore, the results show that 4-aminopyridine is immobilized on surface Fe3O4@SiO2-Pr (Fig. 2d) [58, 59]. Finally, Br3 exhibits a signal between 180 and 200 cm−1, which is not recognizable in the FT‐IR spectrum (Fig. 2e) [60]. The FT-IR spectrum was taken from the recovered catalyst after six cycles. As is shown, the FT-IR of the recovered catalyst dose not changed considerably compared to the virgin catalyst (Fig. 3).
The TGA analysis curve obtained from the nano-catalyst MNPs@SiO2-Pr-AP-tribromide, the first mass reduction in the TGA diagram at 100 ℃ can be attributed to loss of water and organic solvents. The second mass reduction occurs at 250–350 ℃, which is due to the separation of organic parts attached to the nano-catalyst, and the third mass reduction at 350–600 ℃ is related to the decomposition of the nano-catalyst, which indicates that the nano-catalyst is stable up to 350 ℃ (Fig. 4).
In a separate study, to investigate the structural and textural properties of the nano-catalyst MNPs@SiO2-Pr-AP-tribromide, it was analyzed by X-ray in the range of 20–65 ℃ angles. In the XRD model, light reflection peaks at angles of 2θ = 30.10°, 35.00°, 44.10°, 53.00°, 58.00°, and 63.80°confirmed the successful formation of the desired catalyst (Fig. 5).
Using SEM, the size and morphological characteristics of the nano-catalyst MNPs@SiO2-Pr-AP-tribromide were investigated. As shown in (Fig. 6), the obtained SEM micrographs confirm the spherical shape created, and the catalyst particle size is in the range of 35 and 83 nm. Also, the recovered catalyst after six runs had no obvious change in structure, as shown by comparison of the SEM spectra to that of fresh catalyst.
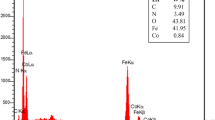
The EDX spectra of the catalyst before and after reaction are shown in (Fig. 7). The EDX spectra of catalyst before and after reaction were almost identical which indicated that there was no obvious change for the catalyst composition after the reaction. The output data of EDX, analysis proves the presence of iron, carbon, oxygen, silicium, nitrogen and, bromine elements in the nano-catalyst MNPs@SiO2-Pr-AP-tribromide.
These observations are also shown by the initial EDS mapping analysis (Fig. 8).
Also, the images obtained from TEM are shown that the nano-catalyst MNPs@SiO2-Pr-AP-tribromide has a uniform spherical morphology of size 30 nm (Fig. 9).
The magnetic properties of nano-catalysts MNPs@SiO2-Pr-AP-tribromide were studied using VSM analysis. The results showed that inserting each layer to the surface of Fe3O4 nanoparticles, it reduces the magnetic property from 60 to 36 emug−1 for the final stage of the catalyst (Fig. 10).
Optimization of the reaction conditions
To select the optimal conditions, models reaction between 2-amino-5-chlorobenzophenone (1 mmol) and pentane 2,4-dion (1 mmol) was investigated by considering the parameters, including different values of catalysts, temperatures, and types of solvents. Initially, the model reaction was performed in the absence of catalyst and solvent, but there was no functional reaction (Table 1, entry 1).

In order to evaluate the performance of the catalyst on the reaction time, the model reaction was performed in different values, including 0.05, 0.08, 0.1, 0.15 g, and without catalyst using ethanol solvent under reflux conditions. The result showed that 0.1 g of the catalyst gave the best product (Table 1, entry 5) and had the weakest performance in the absence of the catalyst (Table 1, entry 2). In addition, increasing the amount of catalyst to 0.15 g does not affect the reaction time and performance. The models reaction was evaluated in the presence of 0.1 g of catalyst in solvents such as EtOH, H2O, EtOH/H2O, DES, DMF, CH3CN, toluene, DCM, and in solvent-free conditions (Table 1, entry 7–14). As can be seen from the results, 0.1 g of catalyst MNPs@SiO2-Pr-AP-tribromide in ethanol solvent was selected as the optimal condition under reflux conditions (Table 1, entry 5). Also, the model reaction was performed at room temperature, and 50, which showed a more favorable result at 50 °C (Table 1, entry15-17).
After selecting the best reaction conditions, we evaluated the protocol in different types of 1,3-diketon and 2-aminoaryl ketone to synthesis quinoline derivatives for the generality and application of this method. The results were summarized as follows (Table 2).
In order to determine the efficiency of the related intermediates MNPs@SiO2-Pr-AP-tribromide, the reaction of the model under reflux conditions was evaluated (Table 3). According to the results obtained, step MNPs@SiO2-Pr-AP-tribromide provided a more favorable result (Table 3, entry 5).
Recycling and reusing of the catalyst
To evaluate the recyclability of catalysts MNPs@SiO2-Pr-AP-tribromide, a two-component compression reaction between acetylacetone and 2-amino-5-chlorobenzophenone in the presence of 0.1 g of catalyst was preferred as the model reaction. After completing the reaction at each run, the catalyst was separated by an external magnet and washed with hot ethanol, and after drying, it was used for the next run. The results showed that the catalytic activity could be repeated up to six times in a row without significantly reducing in product efficiency (Fig. 11).
The proposed reaction mechanism
The suggested plausible mechanism for the synthesis of quinoline derivatives is shown in (Scheme 3). Since MNPs@SiO2-Pr-AP-tribromide contains bromine atoms which are attached to nitrogen atoms, it is probable that they release Br+ in situ. First, 1,3-diketones activated with Br+ react with the amino aryl ketone (I) to from an intermediate of imine (II) by leaving the water. In the next step, Br+ activates the carbonyl group of 2-aminoarylketones, and an intramolecular nucleophilic attack (III) on the carbonyl group occurs, and the quinoline ring is formed by the withdrawal of water.
Conclusion
In summary, the new ionic liquid-based magnetic nanoparticles of pyridinium-tribromide (MNPs@SiO2-Pr-AP-tribromide) was synthesized and used as an effective catalyst in the synthesis of new quinoline derivatives through a one-pot reaction between 2-amino aryl ketones and various ketone compounds. Also, considering the green chemistry, using the dangerous substance bromine on the substrate of 4-aminopyridine and magnetic nano-Fe3O4 was used as a base. This catalyst has a considerable level of Br3− which increases the catalytic activity. Furthermore, other advantages of this catalyst include easy preparation, shortening reaction time and high efficiency of products, easy separation and the possibility of recycling up to six steps without loss of catalytic activity, easy purification of products, solidity, and safety of the catalyst and selectivity.
References
P. Jahanshahi, M. Mamaghani, React. Kinet. Mech. Catal. 130, 955 (2020)
S. Sadjadi, P. Mohammadi, M. Heravi, Sci. Rep. 10, 1 (2020)
K. Nikoofar, S. Khani, Catal. Lett. 148, 1651 (2018)
G. Rahimzadeh, S. Bahadorikhalili, E. Kianmehr, M. Mahdavi, Mol. Divers. 21, 597 (2017)
M.U. Khan, S. Siddiqui, Z.N. Siddiqui, ACS Omega 4, 7586 (2019)
H. Sahebi, E. Konoz, A. Ezabadi, New J. Chem. 43, 13554 (2019)
Z. He, P. Alexandridis, Adv. Colloid Interface Sci. 244, 54 (2017)
M. Yarie, M.A. Zolfigol, Y. Bayat, A. Asgari, D.A. Alonso, A. Khoshnood, RSC Adv. 6, 82842 (2016)
B. Li, Q. Zhang, Y. Pan, Y. Li, Z. Huang, M. Li, H. Xiao, Int. J. Biol. Macromol. 163, 309 (2020)
P. Wasserscheid, W. Keim, Chem. Int. Ed. 39, 3772 (2000)
H. Olivier-Bourbigou, L. Magna, J. Mol. Catal. A. Chem. 182, 419–437 (2002)
R. Gurav, S.K. Surve, S. Babar, P. Choudhari, D. Patil, V. More, S. Hangirgekar, Org. Biomol. Chem. 18, 4575 (2020)
A. Yadav, P. Patil, D. Chandam, S. Jadhav, A. Ghule, S. Hangirgekar, S. Sankpal, J. Mol. Struct. 1245, 130960 (2021)
S. Noori, R. Ghorbani-Vaghei, M. Mirzaei-Mosbat, J. Mol. Struct. 1219, 128583 (2020)
H. Dadhania, D. Raval, A. Dadhania, Polycycl. Aromat. Compd. 41, 440 (2021)
A. Singh, A. Srivastava, M.S. Singh, J. Org. Chem. 83, 7950 (2018)
I. Cano, C. Martin, J.A. Fernandes, R.W. Lodge, J. Dupont, F.A. Casado-Carmona, R. Lucena, S. Cardenas, V. Sans, I. de Pedro, Appl. Catal. B. 260, 118110 (2020)
I. del Hierro, Y. Pérez, M. Fajardo, Mol. Catal. 450, 112 (2018)
B. Paul, B. Bhuyan, D.D. Purkayastha, S.S. Dhar, B.K. Patel, Tetrahedron Lett. 56, 5646 (2015)
L. Wu, Z. Yin, Eur. J. Inorg. Chem. 36, 6156 (2013)
M. Kazemi, L. Shiri, Mini. Rev. Org. Chem. 15, 86 (2018)
C. Teja, F.R.N. Khan, Chem. Asian. J. 15, 4153 (2020)
M.O. Puskullu, I. Celik, M. Erol, H. Fatullayev, E. Uzunhisarcikli, G. Kuyucuklu, Bioorg. Chem. 101, 104014 (2020)
M.J. Momeni, H. Behzadi, P. Roonasi, S.A.S. Sadjadi, S.M. Mousavi-Khoshdel, S.V. Mousavi, Res. Chem. Intermed. 41, 6789 (2015)
L. Wu, C. Yang, B. Niu, F. Yan, Monatsh. Chem. 140, 1195 (2009)
S. Prameela, F.R. Nawaz Khan, Eur. J. Org. Chem. 33, 5394 (2020)
S. Rezayati, M.T. Jafroudi, E.R. Nezhad, R. Hajinasiri, S. Abbaspour, Res. Chem. Intermed. 42, 5887 (2016)
B.P. Reddy, P. Iniyavan, S. Sarveswari, V. Vijayakumar, Chin. Chem. Lett. 25, 1595 (2014)
E. Soleimani, M.M. Khodaei, N. Batooie, S. Samadi, Chem. Pharm. Bull. 58, 212 (2010)
F. Tufail, M. Saquib, S. Singh, J. Tiwari, M. Singh, J. Singh, J. Singh, New J. Chem. 41, 1618 (2017)
W. Hu, W. Yang, T. Yan, M. Cai, Synth. Commun. 49, 799 (2019)
X.M. Li, L. Tang, Z.M. Qian, Y.H. He, Z. Guan, Tetrahedron Lett. 12, 152346 (2020)
S. Gajare, A. Patil, S. Hangirgekar, S. Dhanmane, G. Rashinkar, Res. Chem. Intermed. 46, 2417 (2020)
A. Meléndez, E. Plata, D. Rodríguez, D. Ardila, S.A. Guerrero, L.M. Acosta, J. Cobo, M. Nogueras, A. Palma, synthesis. 52, 1804 (2020)
A.K. Bagdi, S. Santra, M. Rahman, A. Majee, A. Hajra, RSC Adv. 3, 24034 (2013)
G.A. Ramann, B.J. Cowen, Molecules 21, 986 (2016)
R. Ghorbani-Vaghei, S. Akbari-Dadamahaleh, Tetrahedron Lett. 50, 1055 (2009)
A. Das, N. Anbu, P. Varalakshmi, A. Dhakshinamoorthy, S. Biswas, New J. Chem. 44, 10982 (2020)
R.A. Agarwal, A.K. Gupta, D. De, Cryst. Growth. Des. 19, 2010 (2019)
Y.D. Shao, M.M. Dong, Y.A. Wang, P.M. Cheng, T. Wang, D.J. Cheng, Org. Lett. 21, 4831 (2019)
R. Satheeshkumar, K. Shanmugaraj, T. Delgado, J. Bertrand, I. Brito, C.O. Salas, Org. Prep. Proced. Int. 53, 138 (2021)
N.G. Khaligh, T. Mihankhah, M.R. Johan, Polycycl. Aromat. Compd. 40, 1223 (2018)
X.Y. Zhou, X. Chen, L.G. Wang, Synth. Commun. 48, 830 (2018)
A. Singhal, P. Kumari, K. Nisa, Curr. Org. Synth. 16, 154 (2019)
S. Lotfi, A. Nikseresht, N. Rahimi, Polyhedron. 173, 114148 (2019)
D. Katheriya, N. Patel, H. Dadhania, A. Dadhania, J. Iran. Chem. Soc. 18, 805 (2021)
A.T. Garrison, Y. Abouelhassan, H. Yang, H.H. Yousaf, T.J. Nguyen, R.W. Huigens III., MedChemComm. 8, 720 (2017)
H.V. Bailey, M.F. Mahon, N. Vicker, B.V. Potter, ChemistryOpen. 9, 1113 (2020)
A.S. Mali, A.B. Sharma, G.U. Chaturbhuj, Org. Prep. Proced. Int. 52, 297 (2020)
S.V. Ryabukhin, V.S. Naumchik, A.S. Plaskon, O.O. Grygorenko, A.A. Tolmachev, J. Org. Chem. 76, 5774 (2011)
M.K. Barman, A. Jana, B. Maji, Adv. Synth. Catal. 360, 3233 (2018)
J. Xiao, G. Xu, L. Wang, P. Li, W. Zhang, N. Ma, M. Tao, J. Ind. Eng. Chem. 77, 65 (2019)
A. Kharazmi, R. Ghorbani-Vaghei, S. Alavinia, ChemistrySelect 5, 1424 (2020)
R. Ghorbani-Vaghei, N. Sarmast, J. Mahmoodi, Appl. Organomet. Chem. 31, 3681 (2017)
P. Singh, P. Yadav, A. Mishra, S.K. Awasthi, ACS Omega 5, 4223 (2020)
H. Veisi, A. Mirzaei, P. Mohammadi, RSC Adv. 9, 41581 (2019)
A. Khazaei, N. Sarmasti, J. Yousefi Seyf, J. Mol. Liq. 262, 484 (2018)
H. Mohammadi, H.R. Shaterian, Res. Chem. Intermed. 46, 179 (2020)
H. Moghanian, M.A.B. Fard, A. Mobinikhaledi, N. Ahadi, Res. Chem. Intermed. 44, 4083 (2018)
B.S. Ault, L. Andrews, J. Chem. Phys. 64, 4853 (1976)
Acknowledgements
The authors wish to thank Bu-Ali Sina University, Center of Excellence Developmental of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kharazmi, A., Ghorbani-Vaghei, R., Noori, S. et al. Synthesis of multiple quinoline derivatives using novel ionic liquid-based nano-magnetic catalyst (MNPs@SiO2-Pr-AP-tribromide). Res Chem Intermed 48, 1313–1329 (2022). https://doi.org/10.1007/s11164-022-04675-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04675-z