Abstract
A novel heteropolyanion-based Brønsted acidic ionic liquid material [PhBS]3PW12O40, butane mono-sulfoacid-functionalized phenanthrolinum salt of phosphortungstate catalyst (PhBS-PW), was synthesized and well characterized with FTIR, 1H and 13C NMR, electro-spray ionization mass spectrometry (ESI-MS), EDX and TG analysis techniques. The new prepared catalyst was used for the efficient one-pot synthesis of 2H-indazolo[2,1-b]phthalazine-trione derivatives from a three-component condensation reaction between aromatic aldehydes, dimedone, and phthalhydrazide in H2O under thermal conditions. This green approach has several advantages such as short reaction times, clean reaction profiles, and simple experimental and workup procedures. Moreover, the catalyst can be easily recovered and reused at least nine times with only slight reduction in its catalytic activity with no leaching amount of catalyst into the reaction mixture. These economical factors (time, cost, waste, etc.) for this three-component reaction hold promise for the future of organic synthesis.
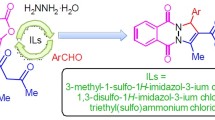
Graphic abstract
In this work we introduced a new efficient Brønsted acidic ionic liquid (BAIL) and then its catalytic activities have been considered in the three-component synthesis of 2H-indazolo[2,1-b]phthalazine-triones via the domino Knoevenagel condensation/Michael addition/intramolecular cyclodehydration sequence in water under reflux condition.












Similar content being viewed by others
References
J.H. Clark, D.J. Macquarrie, Handbook of Green Chemistry and Technology, 1st edn. (Wiley, Oxford, 2002)
M. King, M. Moats, W. Davenport, Sulfuric Acid Manufacture: Analysis, Control and Optimization, 2nd edn. (Elsevier Ltd, Amsterdam, 2013)
P. Wasserscheid, T. Welton, Ionic Liquid in Synthesis, 2nd edn. (Wiley, Weinheim, 2007)
B. Xin, J. Hao, Chem. Soc. Rev. 43, 7171 (2014)
M. Vafaeezadeh, J. Aboudi, M. Mahmoodi Hashemi, RSC Adv. 5, 58 (2015)
M. Keshavarz, B. Karami, A. Zarei Ahmady, A. Ghaedi, H. Vafaei, C. R. Chim. 17, 570 (2014)
A. Zarei Ahmady, F. Heidarizadeh, M. Keshavarz, Synth. Commun. 43, 2100 (2013)
M. Keshavarz, A. Zarei Ahmady, A. Mostoufi, N. Mohtasham, Molecules 22, 1385 (2017)
H. Wang, L. Jia, R. Hu, M. Gao, Y. Wang, Chin. J. Catal. 38, 58 (2017)
W. Zhang, F. Han, J. Tong, C. Xia, J. Liu, Chin. J. Catal. 38, 805 (2017)
O. Goli Jolodar, F. Shirini, M. Seddighi, Chin. J. Catal. 38, 1245 (2017)
H. Hu, Q. Yan, M. Wang, L. Yu, W. Pan, B. Wang, Y. Gao, Chin. J. Catal. 39, 1437 (2018)
P. Zhao, Y. Zhang, D. Li, H. Cui, L. Zhang, Chin. J. Catal. 39, 334 (2018)
R. Turgis, J. Estager, M. Draye, V. Ragaini, W. Bonrath, J.M. Leveque, ChemSusChem 3, 1403 (2010)
S. Safaei, I. Mohammadpoor Baltork, A.R. Khosropour, M. Moghadam, S. Tangestani Nejad, V. Mirkhani, Adv. Synth. Catal. 354, 3095 (2012)
K. Li, L. Chen, H. Wang, W. Lin, Z. Yan, Appl. Catal. A Gen. 392, 233 (2011)
J. Safari, Z. Zarnegar, New J. Chem. 38, 358 (2014)
A. Ying, Z. Li, Y. Ni, S. Xu, H. Hou, H. Hu, J. Ind. Eng. Chem. 24, 127 (2015)
Y.M. Wang, V. Ulrich, G.F. Donnelly, F. Lorenzini, A.C. Marr, P.C. Marr, ACS Sustain. Chem. Eng. 3, 792 (2015)
X. Li, R. Cao, Q. Lin, Catal. Commun. 69, 5 (2015)
M. Vafaeezadeh, M. Mahmoodi Hashemi, RSC Adv. 5, 31298 (2015)
F. Han, L. Yang, Z. Li, Y. Zhao, C. Xia, Adv. Synth. Catal. 356, 2506 (2014)
Z. Wu, Z. Li, G. Wu, L. Wang, S. Lu, L. Wang, H. Wan, G. Guan, Ind. Eng. Chem. Res. 53, 3040 (2014)
H. Ge, Y. Leng, C. Zhou, J. Wang, Catal. Lett. 124, 324 (2008)
Y. Leng, J. Wang, D. Zhu, X. Ren, H. Ge, L. Shen, Angew. Chem. Int. Ed. 48, 168 (2009)
Y. Leng, J. Wang, D. Zhu, Y. Wu, P. Zhao, J. Mol. Catal. A Chem. 313, 1 (2009)
W. Zhang, Y. Leng, P. Zhao, J. Wang, D. Zhu, J. Huang, Green Chem. 13, 832 (2011)
P. Zhao, Y. Leng, M. Zhang, J. Wang, Y. Wu, J. Huang, Chem. Commun. 48, 5721 (2012)
R. Fu, Y. Yang, Z. Chen, W. Lai, Y. Ma, Q. Wang, R. Yuan, Tetrahedron 70, 9492 (2014)
W.H. Zhang, S.S. Liu, P. Liu, J. Xu, B. Xue, X.Y. Wei, Y.X. Li, RSC Adv. 6, 41404 (2016)
X. Duan, G. Sun, Z. Sun, J. Li, S. Wang, X. Wang, S. Li, Z. Jiang, Catal. Commun. 42, 125 (2013)
Y. Leng, P. Jiang, J. Wang, Catal. Commun. 25, 41 (2012)
M.Y. Huang, X.X. Han, C.T. Hung, J.C. Lin, P.H. Wu, J.C. Wud, S.B. Liu, J. Catal. 320, 42 (2014)
R. Fu, Y. Yang, Y. Ma, F. Yang, J. Li, W. Chai, Q. Wang, R. Yuan, Tetrahedron Lett. 56, 4527 (2015)
I. Yavari, R. Hajinasiri, S.Z. Sayyed-Alangi, N. Iravani, Monatshefte Chem. 139, 1029 (2008)
I. Yavari, T. Sanaeishoar, M. Ghazvini, N. Iravani, J. Sulfur Chem. 31, 169 (2010)
N. Iravani, N. Safikhani Mohammadzade, Kh. Niknam, Chin. Chem. Lett. 22, 1151 (2011)
N. Iravani, B. Karami, F. Asadimoghaddam, M. Monfared, N. Karami, J. Sulfur Chem. 33, 279 (2012)
N. Iravani, J. Albadi, S. Varnaseri, Z. Jaberi, N. Karami, M. Khadamati, J. Chin. Chem. Soc. 59, 1567 (2012)
M. Keshavarz, N. Iravani, A. Ghaedi, A. Zarei Ahmady, M. Vafaei Nezhad, S. Karimi, SpringerPlus 2, 64 (2013)
N. Iravani, M. Keshavarz, M. Monfared, F. Hosseini, J. Chin. Chem. Soc. 61, 357 (2014)
S. Nazari, M. Keshavarz, B. Karami, N. Iravani, M. Vafaee-Nezhad, Chin. Chem. Lett. 25, 317 (2014)
N. Iravani, M. Keshavarz, H. Shojaeian Kish, R. Parandvar, Chin. J. Catal. 36, 626 (2015)
M. Keshavarz, N. Iravani, M.H. Ahmadi Azqhandi, S. Nazari, Res. Chem. Intermed. 42, 4591 (2016)
N. Iravani, M. Keshavarz, F. Hosseini, J. Chin. Chem. Soc. 65, 1098 (2018)
N. Iravani, M. Keshavarz, M. Allah-Karampour, J. Sulfur Chem. 39, 414 (2018)
M. Shekouhy, A. Hasaninejad, Ultrason. Sonochem. 19, 307 (2012)
A. Kiasat, A. Moradzadegun, S.J. Saghanejhad, J. Serb. Chem. Soc. 78, 469 (2013)
A. Rostami, B. Tahmasbi, A. Yari, Bull. Korean Chem. Soc. 34, 1521 (2013)
X. Wang, W. Ma, L. Wu, F.L. Yan, J. Chin. Chem. Soc. 57, 1341 (2010)
S. Safaei, I. Mohammadpoor-Baltork, A.R. Khosropour, M. Moghadam, S. Tangestaninejad, V. Mirkhani, Catal. Sci. Technol. 3, 2717 (2013)
A. Hasaninejad, M. Rasekhi Kazerooni, A. Zare, Catal. Today. 196, 148 (2012)
J.M. Khurana, D. Magoo, Tetrahedron Lett. 50, 7300 (2009)
M. Kidwai, R. Chauhan, A. Jahan, Chin. Sci. Bull. 57, 2273 (2012)
B. Mombani Godajdar, M. Etamad, S. Soliemani, Orient. J. Chem. 31, 2131 (2015)
O. Goli-Jolodar, F. Shirini, M. Seddighi, J. Nanosci. Nanotechnol. 18, 591 (2018)
A.B. Atar, S.D. Lee, B.G. Cho, D.W. Cho, Y.T. Jeong, Res. Chem. Intermed. 42, 1707 (2015)
B. Maleki, S. Sedigh Ashrafi, R. Tayebee, Org. Prep. Proced. Int. 49, 542 (2017)
Acknowledgements
The authors gratefully acknowledge to the Gachsaran branch, Islamic Azad University and the Research Council of Yasouj University for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iravani, N., Keshavarz, M. & Parhami, A. Novel SO3H-functionalized phenanthrolinum-phosphotungstate ionic liquid for highly promoted three-component synthesis of 2H-indazolo[2,1-b]phthalazine-triones. Res Chem Intermed 45, 5045–5066 (2019). https://doi.org/10.1007/s11164-019-03875-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03875-4