Abstract
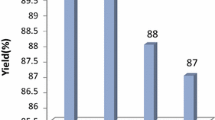
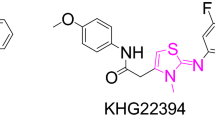
A series of new derivatives of 3-benzimidazolyl-2-aryl thiazolidinones, 4a–j are synthesized via a rapid, one-pot, three-component reaction by using La(NO3)3·6H2O as an efficient catalyst from the reaction of 2-aminobenzimidazole, aromatic aldehydes and thioglycolic acid in ethanol at room temperature. These new compounds were characterized by IR, 1H, 13C NMR and mass spectroscopies. An inexpensive and available catalyst, short reaction time, easy workup, good to excellent yields and nontoxic solvent are the advantages of this reaction.


Similar content being viewed by others
References
L. Weber, Drug Discov. Today 7, 143–147 (2002)
J.C. Menendez, Synthesis 15, 2624 (2006). doi:10.1055/s-2006-949153
M.J. Climent, A. Corma, S. Iborra, RSC Adv. 2, 16–58 (2012)
S. Pola, Signif. Thiazole-based Heterocycles Bioact. Syst. (2016). doi:10.5772/62077
T. Kvernmo, S. Härtter, E. Burger, Clin. Ther. 28, 1065–1078 (2006)
A.K. Jain, A. Vaidya, V. Ravichandran, S.K. Kashaw, R.K. Agraval, Bioorg. Med. Chem. 20, 3378–3395 (2012). and references cited therein
R.V. Patel, S.W. Park, Res. Chem. Intermed. 41, 5599–5609 (2015)
P.D. Neuenfeldt, B.B. Drawanz, G.M. Siqueira, C.R.B. Gomes, S. Wardell, A.F.C. Flores, W. Cunico, Tetrahedron Lett. 51, 3106–3108 (2010)
A.N. Solankee, K.P. Patel, R.B. Patel, Adv. Appl. Sci. Res. 3, 117–122 (2012)
A. Rao, A. Chimirri, S. Ferro, A.M. Monforte, P. Monforte, M. Zappalà, Arkivoc 5, 147–155 (2004)
A. Mobinikhaledi, N. Forughifar, M. Kalhor, M. Mirabolfathy, J. Heterocyclic Chem. 47, 77–80 (2010)
T. Srivastava, W. Haq, S.B. Katti, Tetrahedron 58, 7619–7624 (2002). and references cited therein
N. Foroughifar, S. Ebrahimi, Chin. Chem. Lett. 24, 389–391 (2013)
M.P. Thakare, P. Kumar, N. Kumar, S.K. Pandey, Tetrahedron Lett. 55, 2463–2466 (2014)
H.X. Pang, Y.H. Hui, K. Fan, X.J. Xing, Y. Wu, J.H. Yang, W. Shi, Z.F. Xie, Chin. Chem. Lett. 27, 335–339 (2016)
A.V. Chate, A.G. Tathe, P.J. Nagtilak, S.M. Sangle, C.H. Gill, Chin.Chem. Catal. 37, 1997–2002 (2016)
R.R. Harale, P.V. Shitre, B.R. Sathe, M.S. Shingare, Res. Chem. Intermed. 42, 6695–6703 (2016)
M.P. Thakare, R. Shaikh, D. Tayade, RSC Adv. 6, 28619–28623 (2016)
S.M. Sadeghzadeh, M. Malekzadeh, J. Mol. Liq. 202, 46–51 (2015)
A.K. Yadav, M. Kumar, T. Yadav, R. Jain, Tetrahedron Lett. 50, 5031–5034 (2009)
S.A. Jadhav, M.G. Shioorkar, O.S. Chavan, D.B. Shinde, R.K. Pardeshi, Heterocyclic Lett. 5, 375–382 (2015)
C. Jun, X. Jiangtao, L. Xianjin, Bioorg. Med. Chem. Lett. 15, 267–269 (2005)
K.T. Ashish, M. Anil, Ind. Chem. 45, 489–493 (2006)
E. Carlsson, P. Lindberg, S. Unge, Chem. Br. 38, 42–45 (2002)
M.E. Hassan, M.B. Alaa-eldin, M.B. Sahas, A.A. Farahat, Ind. J. Chem. 49, 1515–1525 (2010)
A.K. Gu¨lgu¨, A. Nurten, Farmaco 58, 1345–1350 (2003)
Y.L. Luo, K. Baathulaa, V.K. Kannekanti, C.H. Zhou, G.X. Cai, Sci. China Chem. 58, 483–494 (2015)
H.Z. Zhang, J.M. Lin, S. Rashid, C.H. Zou, Sci. China Chem. 57, 807–822 (2014)
W. Maxwell, G. Brody, App. Microbial 21, 944–945 (1971)
A.K. G¨ulg¨un, A. Nurten, Turk. J. Chem. 30, 223–228 (2006)
E.S. Lazer, M.R. Matteo, G.J. Possanza, J. Med. Chem. 30, 726–729 (1987)
L. Srikanth, N. Raghunandan, R. Sambasiva, Der Pharma. Chemic. 3, 344–352 (2011)
K. Sreena, R. Ratheesh, M. Rachana, M. Poornima, C. Shyni, Hygei 1, 21–22 (2009)
A. Mobinikhaledi, N. Forughifar, M. Kalhor, Syn. React. Inorg. Met-org. Chem. 39, 509–511 (2009)
K.U. Sadek, F. Al-Qalaf, R.A. Mekheimer, M.H. Elnagdi, Arabian J. Chem. 5, 63–66 (2010)
M.M. Ramla, M.A. Omar, H. Tokuda, H.I. El-Diwoni, Bioorg. Med. Chem. 15, 64896496 (2007)
Z. Kazimierczuk, D. Shugar, Nucleosides Nucleotides 8, 1379–1385 (1989)
M. Kalhor, Org. Chem. Res. 1, 59–65 (2015)
Acknowledgement
We are grateful for the research commute of Chemistry Department of Payame Noor University for providing financial and technical support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalhor, M., Banibairami, S. & Mirshokraie, S.A. A one-pot multi-component reaction for the facile synthesis of some novel 2-aryl thiazolidinones bearing benzimidazole moiety using La(NO3)3·6H2O as an efficient catalyst. Res Chem Intermed 43, 5985–5994 (2017). https://doi.org/10.1007/s11164-017-2974-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-2974-8