Abstract
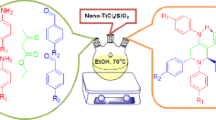
In this work, we have synthesized a novel nanostructured molten salt, 1H-imidazol-3-ium tricyanomethanide {[HIMI]C(CN)3} (1), as an efficient and green protocol-compatible catalyst. This new molten salt has been fully characterized by different analytical techniques, such as FT-IR, 1HNMR, 13CNMR, thermal gravimetric analysis, derivative thermal gravimetric analysis, differential thermal analysis, X-ray diffraction, scanning electron microscopy, and high-resolution transmission electron microscopy. Additionally, the catalytic activity of {[HIMI]C(CN)3} (1, 2 mol%) has been tested in a three-component domino Knoevenagel condensation reaction. A range of structurally diverse aromatic aldehydes (2a–p), malononitrile (3), and 4‐hydroxy‐6‐methyl‐2H‐pyran‐2‐one (4) are tolerated for the synthesis of 2-amino-7-methyl-5-oxo-4-aryl-4,5-dihydropyrano[4,3-b]pyran-3-carbonitrile derivatives (5a–p) under neat conditions at 50 °C. The obtained results have demonstrated that catalyst 1 shows interesting catalytic properties, such as clean reaction profile, cost-effectiveness, and green conditions. Importantly, the aforementioned catalyst is thermally stable with a 171 °C melting point not showing any significant loss in catalytic activity after 7 reaction cycles.








Similar content being viewed by others
Change history
30 June 2020
Since it has been cleared that a fake compound has been sold to us as cyanoform.
References
A. Domling, Chem. Rev. 106, 17 (2006)
A. Domling, I. Ugi, Angew. Chem. Int. Ed. 39, 3168 (2000)
J. Zhu, H. Bienayme, Multicomponent Reactions (Wiley, Weinheim, 2005)
D.M. D’Souza, T.J.J. Mueller, Chem. Soc. Rev. 36, 3169 (2007)
D. Tejedor, F. Garcia-Tellado, Chem. Soc. Rev. 36, 484 (2007)
N.R. Candeias, F. Montalbano, P.M.S.D. Cal, P.M.P. Gois, Chem. Rev. 110, 6169 (2010)
K. Wang, D. Kim, A. Domling, J. Comb. Chem. 12, 111 (2010)
L.F. Tietze, Domino Reactions: Concepts for Efficient Organic Synthesis (Wiley, Weinheim, 2004)
A. Dömling, W. Wang, K. Wang, Chem. Rev. 112, 3083 (2012)
P. Slobbe, E. Ruijter, R.V.A. Orru, MedChemComm 3, 1189 (2012)
T.J.J. Muller, Science of Synthesis: Multicomponent Reactions (Georg Thieme, Stuttgart, 2014)
S. Wang, G.W.A. Milne, X. Yang, I.J. Posey, M.C. Nicklaus, L. Graham, W.G. Rice, J. Med. Chem. 39, 2047 (1996)
A. Mazumder, S. Wang, N. Neamati, M. Niclaus, S. Sunder, J. Chen, G.W.A. Milne, W.G. Rice, J.T.R. Burke, Y. Pommier, J. Med. Chem. 39, 2472 (1996)
L. Pochet, C. Doucet, M. Schynts, N. Thierry, N. Bogetto, B. Pirotte, K.Y. Jiang, B. Masereel, P. Tullio, J. Delarge, M. Reboud-Ravaux, J. Med. Chem. 39, 2579 (1996)
X.S. Wang, J.X. Zhou, Z.S. Zeng, Y.L. Li, D.Q. Shi, S.J. Tu, ARKIVOC xi, 107 (2006)
D. Rajguru, B.S. Keshwal, S. Jain, V.W. Bhagwat, Monatsh. Chem. 144, 1411 (2013)
H. Leutbecher, L.A.D. Williams, H. Rosner, U. Beifuss, Bioorg. Med. Chem. Lett. 17, 978 (2007)
E. Mosaddegh, A. Hassankhani, H. Karimi-Maleh, Mater. Sci. Eng. C 46, 264 (2015)
M. Ghashang, S.S. Mansoor, K. Aswin, Chin. J. Catal. 35, 127 (2014)
E. Mosaddegh, A. Hassankhani, Chin. J. Catal. 35, 351 (2014)
M.N. Elinson, R.F. Nasybullin, G.I. Nikishin, Electrocatalysis 4, 56 (2013)
Z. Hossaini, F. Sheikholeslami-Farahani, S. Soltani, S.Z. Sayyed-Alangi, H. Sajjadi-Ghotabadi, Chem. Heterocycl. Compd. 51, 26 (2015)
N.G. Khaligh, S.B.A. Hamid, Chin. J. Catal. 36, 728 (2015)
E.V. Stoyanova, I.C. Ivanova, D. Heber, Molecules 5, 19 (2000)
D. Rajguru, B.S. Keshwal, S. Jain, Chin. Chem. Lett. 24, 1033 (2013)
N.G. Khaligh, Monatsh. Chem. 145, 1643 (2014)
M. Deetlefs, K.R. Seddon, Chim. Oggi 24, 16 (2006)
M.J. Earle, J.M.S.S. Esperanca, M.A. Gilea, J.N. Canongia Lopes, L.P.N. Rebelo, J.W. Magee, K.R. Seddon, J.A. Widegren, Nature 439, 831 (2006)
M. Kosmulski, J. Gustafsson, J.B. Rosenholm, Thermochim. Acta 412, 47 (2004)
P. Wasserscheid, T. Welton, Ionic Liquids in Synthesis (Wiley, Weinheim, 2003)
P. Wasserscheid, W. Keim, Angew. Chem. Int. Ed. 39, 3773 (2000)
M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, H.G. Kruger, Z. Asgari, V. Khakyzadeh, K. Rostami, J. Org. Chem. 77, 3640 (2012)
M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, H.G. Kruger, Z. Asgari, V. Khakyzadeh, A. Hasaninejad, J. Ind. Eng. Chem. 19, 721 (2013)
M.A. Zolfigol, H. Vahedi, S. Azimi, A.R. Moosavi-Zare, Synlett 24, 1113 (2013)
A.R. Moosavi-Zare, M.A. Zolfigol, O. Khaledian, V. Khakyzadeh, M.D. Farahani, H.G. Kruger, New J. Chem. 38, 2342 (2014)
A.R. Moosavi-Zare, M.A. Zolfigol, V. Khakyzadeh, C. Böttcher, M.H. Beyzavi, A. Zare, A. Hasaninejad, R. Luque, J. Mater. Chem. A 2, 770 (2014)
M.A. Zolfigol, S. Baghery, A.R. Moosavi-Zare, S.M. Vahdat, RSC Adv. 5, 32933 (2015)
M.A. Zolfigol, S. Baghery, A.R. Moosavi-Zare, S.M. Vahdat, H. Alinezhad, M. Norouzi, RSC Adv. 5, 45027 (2015)
M.A. Zolfigol, S. Baghery, A.R. Moosavi-Zare, S.M. Vahdat, H. Alinezhad, M. Norouzi, RSC Adv. 4, 57662 (2014)
M.A. Zolfigol, F. Afsharnadery, S. Baghery, S. Salehzadeh, F. Maleki, RSC Adv. 5, 75555 (2015)
M.A. Zolfigol, S. Baghery, A.R. Moosavi-Zare, S.M. Vahdat, J. Mol. Catal. A Chem. 409, 216 (2015)
H. Sharghi, A. Khoshnood, M.M. Doroodmand, R. Khalifeh, J. Heterocycl. Chem. 53, 164 (2016)
M.A. Zolfigol, M. Yarie, RSC Adv. 5, 103617 (2015)
M.A. Zolfigol, M. Kiafar, M. Yarie, A.A. Taherpour, M. Saeidi-Rad, RSC Adv. 6, 50100 (2016)
M. Yarie, M.A. Zolfigol, Y. Bayat, A. Asgari, D.A. Alonso, A. Khoshnood, RSC Adv. 6, 82842 (2016)
M.A. Zolfigol, M. Yarie, S. Baghery, J. Mol. Liq. 222, 923 (2016)
M.A. Zolfigol, M. Yarie, S. Baghery, Synlett 27, 1418 (2016)
Acknowledgements
We thank Bu-Ali Sina University, Iran National Science Foundation (INSF) (Grant of Allameh Tabataba’i’s Award, Grant Number BN093), National Elites Foundation, University of Alicante (VIGROB-173), and the Spanish Ministerio de Economíay Competitividad (CTQ2015-66624-P) for financial support to our research groups.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zolfigol, M.A., Yarie, M., Baghery, S. et al. 1H-imidazol-3-ium tricyanomethanide {[HIM]C(CN)3} as a nanostructured molten salt catalyst: application to the synthesis of pyrano[4,3‐b]pyrans. Res Chem Intermed 43, 3291–3305 (2017). https://doi.org/10.1007/s11164-016-2826-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2826-y