Abstract
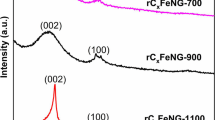
A non-precious metal FeNi electrocatalyst (FeNi/NG) was prepared by a simple solution route called the polyol process for use as the oxygen reduction reaction (ORR) catalyst in polymer exchange membrane fuel cells. The nitrogen-doped graphene (NG) was synthesized from graphite oxide (GO) in a one-pot reactor via thermal annealing of GO-mixed melamine. The obtained NG presents high content of pyridinic-N and quaternary-N types with acceptable activity of ORR in acidic media by XPS and cyclic voltammetry techniques, respectively. The non-precious FeNi alloy nanoparticles (spherical-like nanoparticles mixed with hexagonal plate-like features) were successfully synthesized and well dispersed on the prepared NG by the polyol process confirmed by SEM-BSE and TEM analysis. The XRD and SAED results found FeNi and the carbon phase in the prepared catalysts. Finally, the CV technique shows that the peak potential of FeNi/NG is in the range of 0.12–0.34 V, which is close to that of the commercial Pt/C catalyst (0.35 V). To summarize, the obtained catalyst (FeNi/NG) revealed reliable electrocatalytic properties for ORR in a proton exchange membrane fuel cell cathode.










Similar content being viewed by others
References
W. Vielstich, A. Lamm, H. A. Gasteiger, Handbook of Fuel Cells (Wiley, London, 2003), pp. 449
Z. Chen, D. Higgins, A. Yu, L. Zhang, J. Zhang, Energy Environ. Sci. 4, 9 (2011)
V.S. Bagotsky, A.M. Skundin, Y.M. Volfkovich, Electrochemical Power Sources (Wiley, New York, 2014), pp. 151–170
A. Faur Ghenciu, Curr. Opin. Solid State Mater. Sci. 6, 5 (2002)
R. Othman, A.L. Dicks, Z. Zhu, Int. J. Hydrog. Energy 37, 1 (2012)
R. Bashyam, P. Zelenay, Nature 443, 7107 (2006)
C. Medard, M. Lefevre, J. Dodelet, F. Jaouen, G. Lindbergh, Electrochim. Acta 51, 16 (2006)
S. Ye, A.K. Vijh, Electrochem. Commun. 5, 3 (2003)
G. Wu, M. Nelson, S. Ma, H. Meng, G. Cui, P.K. Shen, Carbon 49, 12 (2011)
M. Huang, Y. Tang, Y. Gong, Y. Chen, Y. Sun, X. Yang, P. Wan, ChemElectroChem 2, 12 (2015)
M. García-Contreras, S. Fernández-Valverde, J.R. Vargas-Garcia, J. Alloys Compd. 434, 522 (2007)
R. Yang, K. Stevens, J. Dahn, J. Electrochem. Soc. 155, 1 (2008)
Y. Shao, J. Sui, G. Yin, Y. Gao, Appl. Catal. B. Environ. 79, 1 (2008)
N.P. Subramanian, X. Li, V. Nallathambi, S.P. Kumaraguru, H. Colon-Mercado, G. Wu, J.-W. Lee, B.N. Popov, J. Power Sources 188, 1 (2009)
K.R. Lee, K.U. Lee, J.W. Lee, B.T. Ahn, S.I. Woo, Electrochem. Commun. 12, 8 (2010)
L. Qu, Y. Liu, J.-B. Baek, L. Dai, ACS Nano 4, 3 (2010)
D. Geng, Y. Chen, Y. Chen, Y. Li, R. Li, X. Sun, S. Ye, S. Knights, Energy. Environ. Sci. 4, 3 (2011)
D. Geng, S. Yang, Y. Zhang, J. Yang, J. Liu, R. Li, T.-K. Sham, X. Sun, S. Ye, S. Knights, Appl. Surf. Sci. 257, 21 (2011)
Z.-H. Sheng, L. Shao, J.-J. Chen, W.-J. Bao, F.-B. Wang, X.-H. Xia, ACS Nano 5, 6 (2011)
R.J. Joseyphus, D. Kodama, T. Matsumoto, Y. Sato, B. Jeyadevan, K. Tohji, J. Magn. Magn. Mater. 310(2), 2393–2395 (2007)
G. Viau, F. Fiévet-Vincent, F. Fiévet, Solid State Ion. 84, 3–4 (1996)
G. Qin, W. Pei, Y. Ren, Y. Shimada, Y. Endo, M. Yamaguchi, S. Okamoto, O. Kitakami, J. Magn. Magn. Mater. 321, 24 (2009)
J. Song, X. Wang, C.-T. Chang, J. Nanomater. 2014, 1 (2014)
A.R. bin Mohdá Yusoff, Nanoscale 7, 16 (2015)
T. Hu, X. Sun, H. Sun, G. Xin, D. Shao, C. Liu, J. Lian, Phys. Chem. Chem. Phys. 16, 3 (2014)
K.N. Kudin, B. Ozbas, H.C. Schniepp, R.K. Prud’homme, I.A. Aksay, R. Car, Nano Lett. 8, 1 (2008)
Z. Lin, G.H. Waller, Y. Liu, M. Liu, C. Wong, Carbon 53, 130 (2013)
Z. Luo, S. Lim, Z. Tian, J. Shang, L. Lai, B. MacDonald, C. Fu, Z. Shen, T. Yu, J. Lin, J. Mater. Chem. 21, 22 (2011)
G. Liu, X. Li, J.-W. Lee, B.N. Popov, Catal. Sci. Technol. 1, 2 (2011)
Y. Holade, N. Sahin, K. Servat, T. Napporn, K. Kokoh, Catalysts 5, 1 (2015)
C. Cheng, F. Xu, H. Gu, New J. Chem. 35, 5 (2011)
B. Blin, F. Fievet, D. Beaupere, M. Figlarz, New J. Chem. 13, 1 (1989)
S. Kattel, G. Wang, J. Mater. Chem. A 36, 1 (2013)
M.L. Rigsby, D.J. Wasylenko, M.L. Pegis, J.M. Mayer, J. Am. Chem. Soc. 137, 13 (2015)
J. Liu, E. Li, M. Ruan, P. Song, W. Xu, Catalysts 5, 3 (2015)
S.C. Ball, S. Hudson, B. Theobald, D. Thompsett, ECS Trans. 1, 8 (2006)
Y. Garsany, O.A. Baturina, K.E. Swider-Lyons, S.S. Kocha, Anal. Chem. 82, 15 (2010)
C. Wang, D. Van der Vliet, K.L. More, N.J. Zaluzec, S. Peng, S. Sun, H. Daimon, G. Wang, J. Greeley, J. Pearson, A.P. Paulikas, Nano Lett. 11, 3 (2010)
I. Takahashi, S.S. Kocha, J. Power Sources 195, 19 (2010)
A.H.M. Videla, S. Ban, S. Specchia, L. Zhang, J. Zhang, Carbon 76, 511 (2014)
Y. Holade, N.E. Sahin, K. Servat, T.W. Napporn, K.B. Kokoh, Catalysts 5, 1 (2015)
H. Ghanbarlou, S. Rowshanzamir, B. Kazeminasab, M.J. Parnian, J. Power Sources 273, 981 (2015)
Acknowledgements
This work was supported by the Research, Development, and Engineering (RD&E) fund through The National Nanotechnology Center (NANOTEC), The National Science and Technology Development Agency (NSTDA), Thailand (P-10-10820). Grants from the Center for Innovation in Chemistry, Postgraduate Education and Research Program in Chemistry (PERCH-CIC), and the National Research University Project of Thailand (NRU) are also acknowledged. Finally, the Graduate School of Chiang Mai University is also thanked for the general support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sirirak, R., Jarulertwathana, B., Laokawee, V. et al. FeNi alloy supported on nitrogen-doped graphene catalysts by polyol process for oxygen reduction reaction (ORR) in proton exchange membrane fuel cell (PEMFC) cathode. Res Chem Intermed 43, 2905–2919 (2017). https://doi.org/10.1007/s11164-016-2802-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2802-6