Abstract
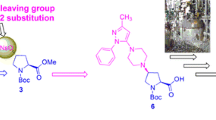
(S)-N-Boc-3′-hydroxyadamantylglycine (I) is an important intermediate of saxagliptin for type 2 diabetes mellitus (T2DM). It was prepared from 1-adamantanecarboxylic acid(1) via mild reaction with sulfuric acid/nitric acid, VHA reagent (SOCl2/DMF) and sodium diethyl malonate, then was treated with hydrolysis, decarboxylation, alkalization and oxidation to give 2-(3-hydroxy-1-adamantyl)-2-oxoacetic acid (4), then through oximation, reduction and (Boc)2O protection to give the N-Boc-3′-hydroxyadamantylglycine(6), then was treated with quinidine to get (S)- N-Boc-3′-hydroxyadamantylglycine(I) and quinine to get (R)–N-Boc-3′-hydroxyadamantylglycine(II). Finally, Compound II was racemized by dicyclohexylcarbodiimide (DCC) and sodium hydride (NaH) to afford compound 6. In this route, the overall yield of preparing compound I was about 35 % and the enantiomeric excess (ee) reach to 99 %. This route provided a novel idea for the preparation of (S)-N-Boc-3′-hydroxyadamantylglycine.





Similar content being viewed by others
References
International Diabetes Federation-Key Findings, IDF Diabetes Atlas Seventh Edition Poster Update 2015. http://www.diabetesatlas.org/(2015)
M.L. Mohler, Y. He, Z. Wu et al., Recent and emerging anti-diabetes targets [J]. Med. Res. Rev. 29(1), 125–195 (2009)
B.M. Squibb, ONGLYZA (saxagliptin) Tablets Prescribing Information (Bristol-Myers Squibb, Princeton, 2011)
V.A. Soloshonok, C. Cai, V.J. Hruby, Stereochemically defined C-substituted glutamic acids and their derivatives. 1. An efficient asymmetric synthesis of (2S,3S)-3-methyl- and -3-trifluoromethylpyroglutamic acids[J]. Tetrahedron 55, 12031–12044 (1999)
J.L. Aceña, A.E. Sorochinsky, V.A. Soloshonok, Recent advances in asymmetric synthesis of a-(trifluoromethyl)-containing amino acids. Synthesis 44, 1591–1602 (2012)
P. Etayo, A. Vidal-Ferra´n, Rhodium-catalyzed asymmetric hydrogenation as a valuable synthetic tool for the preparation of chiral drugs. Chem. Soc. Rev. 42, 728–754 (2013)
J. Wang, X. Liu, X. Feng, Asymmetric strecker reactions. Chem. Rev. 111, 6947–6983 (2011)
K. Undheim et al., The Scho¨llkopf chiron and transition metal mediated reactions, a powerful combination for stereoselective construction of cyclic a-quaternary-a-amino acid derivatives. Amino Acids 34, 357–402 (2008)
A.E. Sorochinsky et al., Asymmetric synthesis of a-amino acids via homologation of Ni(II) complexes of glycine Schiff bases. Part: 1 alkyl halide alkylations. Amino Acids 45, 691–718 (2013)
A.E. Sorochinsky et al., Asymmetric synthesis of a-amino acids via homologation of Ni(II) complexes of glycine Schiff bases. Part 2: Aldol, Mannich addition reactions, deracemization and (S) to (R) interconversion of a-amino acids. Amino Acids 45, 1017–1033 (2013)
J.L. Aceña et al., Asymmetric synthesis of a-amino acids via homologation of Ni(II) complexes of glycine Schiff bases Part 3 Michael addition reactions and miscellaneous transformations. Amino Acids 46, 2047–2073 (2014)
Y. Nian et al., Recyclable ligands for the non-enzymatic dynamic kinetic resolution of challenging a-amino acids. Angew. Chem. Int. Ed. 54, 12918–12922 (2015)
T.C. Vu, D.B. Brzozowski, R. Fox, Methods and compounds for producing dipeptidyl peptidase IV inhibitors and intermediates thereof [P]. WO 2004/052850 A2 (2004)
R.L. Hanson, S.L. Goldberg, D.B. Brzozowski, Preparation of an amino acid intermediate for the dipeptidyl peptidase IV inhibitor, saxagliptin, using a modified phenylalanine dehydrogenase [J]. Adv. Synth. Catal. 349, 1369–1378 (2007)
D.J. Augeri, J.A. Robl, D.A. Betebenner et al., Discovery and preclinical profile of saxagliptin (BMS-477118): a highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes [J]. J. Med. Chem. 48, 5025–5037 (2005)
D Benito-Garragori, W Felzmann, K Knepper et al., Process for the reductive amination of α-keto carboxylic acids. WO 2012/028721 A1 (2012)
Y. Chen, A. Wang, Z. Tao et al., A facile synthesis of saxagliptin intermediate N-Boc-3′-hydroxyadamantylglycine [J]. Res. Chem. Intermed. 41, 4113–4121 (2015)
A. Wang, Y. Deng et al., A convenient method for the synthesis of (S)-N-boc-3-hydroxyadamantylglycine: a key intermediate of saxagliptin [J]. Lett. Org. Chem. 11, 627–663 (2014)
T.C. Vu, D.B. Brzozowski, R. Fox et al. Methods and compounds producing dipeptidyl peptidase IV inhibiors and intermediates thereof. WO 2004/052850 A2 (2004)
V Reddy, R Mitra, A Kumar, P. Ramakrishna, A process for industrial preparation for [(S)-N-tert butoxycarbonyl-3-hydroxy]adamantylglycine. WO 2014/057495 A1 (2014)
J. Dong, Y. Gong, J. Liu, X. Chen, X. Wen, H. Sun, Synthesis and biological evaluation of all eight stereoisomers of DPP-IV inhibitor saxagliptin. Bioorg. Med. Chem. 22, 1383–1393 (2014)
M. Politino, M.M. Cadin, P.M. Skonezny, J.G. Chen, Process for preparing dipeptidyl IV inhibitors and intermediates therefor [P]. WO 2005106011 (2005)
S.A. Savage, G.S. Jones, S. Kolotuchin, S.A. Ramrattan, T. Vu, R.E. Waltermire, Preparation of saxagliptin, a novel DPP-IV inhibitor. Org. Process Res. Dev. 13(6), 1169–1176 (2009)
J.K. Li, H.R. Zhou, J. Peng et al., Synthesis of 2-(3-hydroxy-1-adamantyl)-2-glyoxylic Acid. Chin. J. Pharm. 43(4), 251–253 (2012)
Y. Feng, Y.J. Chen, J. Peng et al., Optimization of the synthesis process of 2- (3- hydroxy- 1- adamantyl)- 2-oxoacetic acid by the central composite design- response surface methodology. Chem. Res. Appl. 2, 194–199 (2013)
S.L. Ferreira, R.E. Bruns, J.M. David et al., Statistical designs and response surface techniques for the optimization of chromatographic systems. J. Chromatogr. A 1158(1–2), 2–14 (2007)
W Thorsten, Racemisation of (R)-N-BOC-3-hydroxyadamantyl glycine [P]. WO 2011/117393 A1 (2011)
V.V. Pozdnyakov, I.K. Moiseev, Synthesis of 3-R-1-acetyladamantanes by substitution in 3-chloro- and 3-hydroxy-1-acetyladamantanes. Russ. J. Org. Chem. 39(5), 739–741 (2003)
J.D. Godfrey Jr., R.T. Fox et al., Novel 1,4-homofragmentation via an α-lactone [J]. J. Org. Chem. 71, 8647–8650 (2006)
Acknowledgments
We deeply appreciate the financial support of this research by a grant from the graduate scientific research and innovation projects of Chongqing Education Committee (No. CYS15142). We are also grateful for all that Chongqing Medical University and Chongqing Research Center for Pharmaceutical Engineering have done for accomplishing this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, J., Jiang, X., Gan, R. et al. A facile and economic method for the synthesis of (S)-N-Boc-3′-hydroxyadamantylglycine. Res Chem Intermed 42, 5709–5721 (2016). https://doi.org/10.1007/s11164-015-2398-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2398-2