Abstract
Oophagous reproduction (i.e., consumption of unfertilized ova in utero) in sharks has been hypothesized to result in fewer but larger pups relative to those produced by viviparous sharks with different modes of maternal nutrient transfer. We compared pup and litter sizes reported in the literature for 106 shark species with lecithotrophic viviparity, oophagy, and placental viviparity as methods of maternal nutrient transfer during pregnancy. Using a suite of permutational tests, we accounted for the effect of maternal size to test whether oophagous strategies do indeed result in larger pups and smaller litters relative to sharks with lecithotrophic and placental viviparous reproduction. Our results demonstrated that litter size was significantly reduced in species with oophagous reproduction relative to sharks with lecithotrophic and placentally viviparous reproduction. Further, the influence of oophagous reproduction on pup length was more variable, and generally pup length of oophagous species was only larger than sharks with lecithotrophic viviparous reproduction. However, when maternal investment was expressed as litter mass (minimum pup mass by litter size), the effect of oophagy was neutralized. We found further evidence that pup length at birth was directly modulated by litter size and habitat, suggesting pup length at birth may also be adapted to conditions at nursing grounds. Our study supports the hypothesis that both placentally viviparous and lecithotrophic viviparous species maximize their reproductive fitness by allocating nutrients to larger litters of pups, whereas oophagous species maximize reproductive fitness through smaller litters of pups that may be well adapted to their nursing grounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sharks represent a diverse array of more than 500 species of cartilaginous fishes that have adapted to a multitude of climates, habitats, and ecological niches (Weigmann 2016). Sharks are K-selected species, as their life histories typically comprise long lifespans with slow development to maturity and low fecundity (Wourms 1977; Dulvy and Forrest 2010). Since sharks do not provide postnatal care to their young, they rely on other reproductive strategies requiring high maternal investment to improve the survivorship of their offspring (Compagno 1984; Cortés 2000). The majority of sharks are viviparous, giving live birth to offspring that are fully developed and prepared for life after parturition (Wourms 1977; Musick and Ellis 2005). Viviparous sharks have evolved a broad range of reproductive modes with variation in nutrient transfer from the female to the developing embryos, with reproductive strategies defined by how embryos are nourished during pregnancy.
Reproductive modes in viviparous sharks exist on a continuum of maternal investment, with lecithotrophy and matrotrophy representing opposite extremes of nutrient transfer during pregnancy (Musick and Ellis 2005; Buddle et al. 2019). All viviparous sharks are initially lecithotrophic, supplying individual embryos with a finite reserve of nutrients via yolk while developing in utero (Wourms 1977). For some viviparous species, lecithotrophy is the sole mechanism for nutrient transfer during the entirety of development (hereafter, “lecithotrophic viviparity”; observed in every extant order of sharks except Heterodontiformes and Lamniformes, Musick and Ellis 2005). Embryos of lecithotrophic viviparous sharks will eventually lose c. ≥ 20% of their initial organic mass by the end of the developmental period as their individual nutrient stores are metabolized (Hamlett et al. 2005; Huveneers et al. 2011). In other cases, multiple mechanisms of nutrient transfer will occur for a single species over the course of embryonic development. For species with matrotrophic viviparity, additional extravitelline nutrients are supplied to their offspring at different stages of gestation (Musick and Ellis 2005; Ostrovsky et al. 2016).
Matrotrophy via embryonic consumption of histotroph is the least recently evolved method of nutrient transfer during pregnancy (Musick and Ellis 2005) wherein embryos receive additional nutrition from uterine secretions (e.g., mucus in muccoid histotrophy and histophagy, Hamlett 1989; egg capsule fluids in embryotrophy, Castro et al. 2016; lipid-rich uterine fluids in lipid histotrophy, Sato et al. 2016). Histotrophy has been observed in species within the superorder Squalomorphii (Hexanchiformes, Squaliformes, Squantiformes, and Pristiophoriformes) and can exist in combination with other forms of matrotrophy, such as within placentally viviparous members of the order Carcharhiniforms (Musick and Ellis 2005), and in the oophagous white shark Carcharodon carcharias (order: Lamniformes, see below). The most recently evolved form of matrotrophy in sharks is placental viviparity, where each embryo develops a yolk sac placenta that facilitates nutrient transport from maternal uterine tissues to the embryos after the depletion of their yolk stores (Wourms 1981). The majority of placentally viviparous sharks remain within their egg capsules during development with nutrient transport occurring across the capsule membrane (Buddle et al. 2019, 2021). This reproductive mode has only been observed within five families, all belonging to the order Carcharhiniformes (Wourms and Lombardi 1992; Conrath and Musick 2012).
Matrotrophic oophagy (hereafter, “oophagy”) is an adaptation in which developing embryos consume unfertilized ova during gestation after the depletion of its yolk stores. Oophagy is thought to occur in all sharks belonging to the order Lamniformes (Gilmore 1993; Gilmore et al. 2005), as well as in the carcharhiniform family Pseudotriakidae (Yano and Musick 1992; Conrath and Musick 2012) and in the orectolobid tawny nurse shark Nebrius ferrugineus (Teshima et al. 1997). Lamnoid oophagy involves continued ovulation during the gestation period, which provides embryos with unfertilized ova during pregnancy which are located and consumed after the embryo exits its egg capsule (Musick and Ellis 2005). In carcharhiniform oophagy, a finite number of ova are provided to each embryo that are contained within its associated egg capsule (Musick and Ellis 2005). Orectolobiform oophagy more closely resembles lamnoid oophagy, with embryonic N. ferrugineus having been found to be highly motile in utero, moving between the left and right uterus, likely as a behavioral adaptation to locate ova (Tomita et al. 2019). The embryos of at least one oophagous species, C. carcharias, have been observed to engage in lipid histotrophy, the consumption of lipid-rich uterine secretions. This behavior likely precedes the oophagous phase (Sato et al. 2016). Embryos of at least two lamniform species, sand tiger shark Carcharias taurus and shortfin mako shark Isurus oxyrinchus, have been observed to perform intrauterine cannibalism of their siblings (i.e., adelphophagy) in addition to oophagy (Gilmore 1993; Joung and Hsu 2005). However, adelphophagy is not ubiquitous in Lamniformes. In contrast to the likely opportunistic occurrence of adelphophagy of runts and dead embryos with the benefit of additional nutrients observed in I. oxyrinchus (Castro 2013), embryos of C. taurus actively hunt and feed on their siblings as adelphophagy is required in order to obtain the nutrients necessary to complete embryonic development before parturition (Gilmore et al. 2005; White 2007). Once the largest C. taurus embryo in each uterine horn has reached a size of c. 100 mm and exhausted its yolk reserves, it will begin to depredate its smaller siblings (Gilmore et al. 2005). C. taurus embryos have been noted to favor cannibalism over the consumption of unfertilized ova, although once a single embryo remains within a uterine horn it will engage in oophagy (Musick and Ellis 2005).
Oophagous reproductive modes are assumed to benefit sharks by allowing them to invest more energy into fewer, larger offspring. Maternal investment should be optimized to maximize offspring survival while minimizing the energetic cost to the parent (Williams 1966). Therefore, for oophagous species, the potential trade-off between litter size and the amount of nutrients or space the mother is able to provide each embryo during pregnancy may result in larger pups that may have lower predation risk after they are born, while also increasing the potential for pups to select from a wider variety of prey in their environment (Gilmore et al. 2005; Parsons et al. 2008; Oliveira et al. 2010).
To our knowledge, the effect of oophagy on pup size has not been quantified using modern datasets. Currently, more than one-third of shark species are threatened with extinction (Dulvy et al. 2021) and understanding reproduction will be essential to guide conservation strategies and gauge the potential for species recovery. Herein, we tested the hypotheses that oophagous reproduction result in larger pups and smaller litters in a comparison of oophagous viviparous sharks to viviparous sharks that are lecithotrophic or placentally viviparous (Compagno 1977; de Carvalho 1996). We also explored relationships between fecundity, gestation period, and maternal and pup sizes (expressed both in terms of length and mass) to determine how reproductive variables are expressed along a maternal size continuum within each reproductive mode. Additionally, we quantified the relative importance of ecology (e.g., adult habitat utilization) on pup sizes. We predicted that oophagous sharks are born at larger sizes relative to maternal size than viviparous lecithotrophic species, and that oophagous sharks will have smaller litters than sharks using other viviparous strategies.
Methods
Data collection
We reviewed the available peer-reviewed and grey (e.g., books, government reports, theses, and online databases) literature on shark size and reproduction. We limited data collection to shark species belonging to the orders Squaliformes (n = 16 spp., c. 12% of extant species), Hexanchiformes (n = 5 spp., c. 71% of extant species), Carcharhiniformes (n = 60 spp., c. 30% of extant species), Lamniformes (n = 13 spp., c. 87% of extant species), and Orectolobiformes (n = 12 spp., c. 28% of extant species; Table 1). We selected sharks based on their reproductive mode, with the goal of comparing oophagous species with lecithotrophic (i.e., lower maternal investment) and placentally viviparous (i.e., higher maternal investment) species across a comparable range of body sizes. Although some species of non-oophagous, non-placentally viviparous sharks also contribute a substantial energetic investment toward the production of extravitelline nutrients for their embryos (e.g., embryotrophic tiger sharks, Galeocerdo cuvier), others contribute much less and to the degree that these species were initially misidentified as lecithotrophic (Conrath and Musick 2012; Cotton et al. 2015). As there are insufficient available data to reconstruct the broad continuum of maternal energy investment for our analyses, viviparous species thought to rely primarily on any form of histotrophy during embryo development were not considered in this study. Whenever possible, we included replicates (i.e., any additional observation of size or reproductive variables for the same species, regardless of sampling location; range of replicates per species = 1–13, median replicates per species = 2). We recorded the sampling location of each study and classified it into larger geographic regions (hereafter, “ecoregion”; see Supplemental Methods).
From our literature review, we recorded adult female and pup sizes (in meters, m, and kilograms, kg). Specifically, we recorded maximum female length, minimum female length at maturity (the length at which 50% of females belonging to a population are sexually mature [L50]), minimum and maximum pup length, maximum female mass, female mass at maturity, and minimum pup mass. When length values were not associated with an observed mass (i.e., the mass of the specimen was not reported), mass was estimated using minimum pup length, minimum female length at maturity, and maximum female length values in length–weight functions from primary and grey literature. For some species, length–weight functions differ with ecoregions (e.g., pelagic thresher Alopias pelagicus); therefore, we used ecoregion-specific length–weight functions to compute mass when we had corresponding lengths from the same ecoregion (see Supplemental Methods for more detail). For species that are widely distributed but data limited (i.e., basking shark Cetorhinus maximus, longfin mako shark I. paucus, megamouth shark Megachasma pelagios, smalltooth sand tiger shark Odontaspis ferox, whitenose shark Nasolamia velox, sharpnose sevengill shark Heptranchias perlo, and whale shark Rhincodon typus), it was not possible to obtain biological information regionally, and instead are represented by all known data at the species level (Appendices 1–4).
Additionally, we collected data on shark reproductive biology. We recorded reproductive mode (i.e., oophagic, lecithotrophic, and placentally viviparous), median and maximum litter size, and gestation period (in months). For litter sizes, we avoided recording minimum reported litter size because these values may be artificially biased due to the occurrence of stress-induced abortion during landing (Adams et al. 2018). Because we had relatively fewer observations of gestation period, we averaged gestation period at the species level for analysis.
Lastly, we collected habitat utilization and depth range data for each species. We defined habitat use as neritic (coastal species utilizing areas from the coast up to the continental shelf), oceanic (offshore species utilizing areas beyond the shelf), or both (species utilizing both coastal and oceanic areas). We defined depth range as epipelagic (maximum reported depths are ≤ 200 m), mesopelagic (maximum reported depths are 201–1000 m), and bathypelagic (maximum reported depths are > 1000 m). Habitat utilization and depth range data were obtained from the primary literature, Fish Base (Froese and Pauly 2016), and the International Union for Conservation of Nature (IUCN 2017).
Statistical analyses
All analyses were performed in the statistical computing environment R, ver. 4.1.1 (R Core Development Team 2021). In our analyses, we (1) determined whether pup and litter sizes were independent of maternal size, (2) tested for mean differences in pup and litter size ratios between reproductive modes, and (3) assessed the relative importance of biological (i.e., size and reproductive variables) and environmental variables in regulating pup size ratios using the methods described below.
Because larger shark species tend to produce larger pups and that maternal size can influence litter size (Cortés 2000), we examined the strength of these associations in our dataset. We used Kendall’s rank correlation coefficient (τ, used as our data were not normally distributed; Croux and Dehon 2010) and tested for significant associations between pup size, litter size, and female size based on the average values for each species. We also tested for an association in maximum female length and gestation period using Kendall’s rank correlation. Significant correlations (α ≤ 0.05) indicated that pup or litter sizes were not independent of maternal size and thus warranted analysis of size ratios to account for effects of maternal size. Based on our findings, we then generated four ratios of the length of pups relative to the length of the mother (Table 2a), four ratios of litter size relative to the length of the mother (Table 2b), and four ratios of pup mass relative to the mass of the mother (Table 2c) to be used in the next steps of our analyses. For maximum length-based ratios, total length (TL) was used.
Next, we tested whether oophagous reproductive modes (i.e., matrotrophic viviparity with oophagy, as observed in species of Lamniformes, two species of Carcharhiniformes and one species of Orectolobiformes) result in larger pups and smaller litters. We detected severe violations of normality and homogeneity of variances for our size ratios that could not be resolved through transformations. Thus, we employed a permutation-based approach to compare the size ratios between reproductive modes (see Supplemental Methods). This analytical approach creates a custom null distribution for each comparison and allows us to quantify the probability that a difference between reproductive modes would occur if there was no true difference (Hooton 1991). In this analysis, we used the mean difference for each size ratio (comparing oophagy-lecithotrophic viviparity and oophagy-placental viviparity separately) as our critical effect size. For each ratio, we generated 10,000 permutations of our data and calculated P-values calculated as follows:
where b is the number of permutations with a mean difference equal to or more extreme than the critical effect size, and m is the total number of permutations (Phipson and Smyth 2010). Because pup sizes were hypothesized to be larger and litter size presumed to be smaller among species with oophagous reproductive strategies, all P-values calculated were one-tailed. We ran analyses with and without species belonging to the family Alopiidae (due to unique tail morphology) and R. typus (due to their massive size) to determine if any of our results were influenced (skewed) by body size of these species.
Since reproductive mode could consistently explain differences in litter size ratios but not pup size ratios (see Results), we then quantified the relative importance of biological and environmental variables on pup size at birth. To employ a conservative approach in our analyses, we used the ratio of minimum pup length and maximum female length (Table 2a) as the dependent variable. Because we found evidence that litter size was regulated by oophagy, we selected the maximum litter size and maximum female length ratio (Table 2b) as a biological explanatory variable. Additionally, we included depth and habitat as ecological explanatory variables. To simplify these analyses by reducing degrees of freedom, we reduced the number of depth categories to two (epipelagic: depth ≤ 200 m; meso/bathypelagic: depth > 200 m). We did not consider gestation period here, as we detected significant correlations with maximum female length (see Results). We included all two- and three-way interactions among possible explanatory variables in our analyses. We used bootstrapped stepwise linear regressions in the R package bootStepAIC (Rizopoulos 2009) with 10,000 bootstrap samples (sampled with replacement) to fit the linear models. We summarized the percentage of times each variable was selected as an independent predictor in our most parsimonious model for each bootstrap sample, as well as the mean parameter estimates with confidence intervals.
Results
Description of data
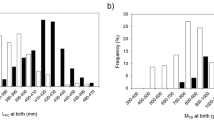
On average, minimum pup length (m) was greatest for oophagous species (x̄ ± SE: 0.99 ± 0.05 m, n = 55 observations of 16 species) followed by placentally viviparous (x̄ ± SE: 0.54 ± 0.01 m, n = 240 observations of 58 species) and lecithotrophic species (x̄ ± SE: 0.30 ± 0.01 m, n = 86 observations of 32 species; Fig. 1a). Conversely, maximum litter size (pups per litter) was smallest among species with oophagy (x̄ ± SE: 5.63 ± 0.64 pups, n = 49 observations of 14 species), with placentally viviparous (x̄ ± SE: 14.0 ± 1.00 pups, n = 200 observations of 56 species) and lecithotrophic species (x̄ ± SE: 40.8 ± 9.12 pups, n = 71 observations of 27 species) having larger litters (Fig. 1b). When considering maximum female length (m), oophagous species were on average larger (x̄ ± SE: 3.98 ± 0.24 m, n = 55 observations of 16 species) than placentally viviparous (x̄ ± SE: 2.17 ± 0.06 m, n = 241 observations of 58 species) and lecithotrophic species (x̄ ± SE: 2.75 ± 0.46 m, n = 86 observations of 32 species; Fig. 1c). However, maximum female mass (kg) of lecithotrophic species were greater (x̄ ± SE: 6267.1 ± 2525.7 kg, n = 45 observations of 21 species) than both oophagous (x̄ ± SE: 809.0 ± 302.2 kg, n = 25 observations of 11 species) and placentally viviparous sharks (x̄ ± SE: 88.7 ± 7.4 kg, n = 158 observations of 46 species; Fig. 1d).
Correlations
There were strong, positive correlations between minimum pup length and maximum female length, which were significant for all three reproductive modes (oophagy: τ = 0.70, P < 0.001, n = 16; placental viviparity: τ = 0.67, P < 0.001, n = 58; lecithotrophy: τ = 0.64, P < 0.001, n = 32; Fig. 2a), and which were nearly identical to the values for minimum pup length and minimum length at maturity (oophagy: τ = 0.73, P < 0.001, n = 15; placental viviparity: τ = 0.68, P < 0.001, n = 52; lecithotrophy: τ = 0.56, P < 0.001, n = 30; Fig. 2b). There was no significant correlation between minimum pup length and maximum female mass for oophagous species (τ = 0.35, P = 0.16, n = 11), although significant relationships were found for placentally viviparous (τ = 0.68, P < 0.001, n = 46) and lecithotrophic species (τ = 0.72, P < 0.001, n = 21; Fig. 2c).
When examining litter size ratios, we found significant correlations between maximum litter size and maximum female length for lecithotrophic (τ = 0.74, P < 0.001, n = 27) and placentally viviparous species (τ = 0.43, P < 0.001, n = 56) but not for oophagous species (τ = 0.19, P = 0.37, n = 14; Fig. 2d), with similar results when considering minimum length at maturity (lecithotrophy: τ = 0.77, P < 0.001, n = 26; placental viviparity: τ = 0.36, P < 0.001, n = 52; oophagy: τ = 0.26, P = 0.24, n = 13; Fig. 2e). When considering the correlation between maximum litter size and maximum female mass, we again found the strongest correlations for lecithotrophic (τ = 0.82, P < 0.001, n = 18) and placentally viviparous species (τ = 0.30, P = 0.004, n = 43) but not for oophagous species (τ = 0.39, P = 0.14, n = 10; Fig. 2f).
When considering a possible relationship of minimum pup length/maximum female length and maximum litter size, we found strong, negative correlations across all reproductive modes (lecithotrophy: τ = -0.67, P < 0.001, n = 27; placental viviparity: τ = -0.59, P < 0.001, n = 56; oophagy: τ = -0.49, P = 0.02, n = 14; Fig. 2g) which was also similar when considering minimum pup length/minimum length at maturity (lecithotrophy: τ = -0.71, P < 0.001, n = 26; placental viviparity: τ = -0.49, P < 0.001, n = 52; oophagy: τ = -0.58, P = 0.008, n = 13; Fig. 2h). The effect of maternal length on pup length and litter sizes (i.e., minimum pup length/maximum female length and maximum litter size/maximum female length) were negatively correlated for lecithotrophic (τ = -0.51, P < 0.001, n = 27) and placentally viviparous species (τ = -0.27, P = 0.003, n = 56), but not for oophagous species (τ = -0.08, P = 0.75, n = 14; Fig. 2i).
For all reproductive modes, we found significant correlations of mean gestation period and maximum female length. For both oophagous and placentally viviparous species, gestation period was greater for larger females (oophagy: τ = 0.65, P = 0.03, n = 8; placental viviparity: τ = 0.21, P = 0.04, n = 48), though the opposite was true for lecithotrophic species (τ = -0.49, P = 0.04, n = 11).
Permutational analysis
We found that pups length ratios of oophagous species were consistently larger than species with lecithotrophic reproductive modes (Fig. 3a). When compared to placentally viviparous species, pup length ratios of oophagous species were greater only when length ratios were based on maximum pup length and female length at maturity (Fig. 3a). In fact, by accounting for maternal length, some mean length ratios of species with oophagous reproductive modes were exceeded by those of placentally viviparous species, although these differences were non-significant (Table 3a). When assessing litter size ratios, we found that all significantly differed between reproductive modes (Fig. 3b), with oophagous species having smaller litters than those of species with other reproductive modes for all comparisons (Table 3b). For mass ratios, we found some mixed results; the minimum pup mass/maximum female mass ratio significantly differed between the oophagous and non-oophagous reproductive modes, though this was less apparent when maternal size was expressed as minimum mass at maturity (Fig. 3c, Table 3c). When reproductive output was expressed as litter mass (minimum pup mass × maximum litter size), we found no effect of reproductive mode when controlling for maximum female mass; however, we did detect significantly greater litter mass in placentally viviparous species over oophagous species when accounting for female mass at maturity (Fig. 3c, Table 3c).
Permutational mean differences for oophagous and lecithotrophic (black points and error bars) and oophagous and placentally viviparous (blue points and error bars) species for each of the (a) pup length ratios, (b) litter size ratios, and (c) pup mass ratios analyzed. Error bars represent the range of permutational mean differences. Triangles represent the observed mean difference between reproductive modes. Asterisks indicate significance: *P < 0.05, **P < 0.01, ***P < 0.001
The exclusion of the family Alopiidae nor R. typus did not change the significance levels of any of our permutation comparisons.
Bootstrap modelling
The stepwise bootstrap modelling exercise most frequently selected the maximum litter size ratio (maximum litter size/maximum female length) and habitat as the two variables that best explained the minimum pup length/maximum female length ratio (Table 4). The most parsimonious model identified included the maximum litter size/maximum female length ratio and habitat. Larger litter size ratios reduced pup length ratio, and larger pup length ratios were associated with neritic habitats (Table 5).
Discussion
Oophagous reproduction has been hypothesized to increase individual fitness (relative to non-oophagous reproductive modes) by allowing sharks to invest more energy into fewer, larger offspring (Gilmore et al. 2005; Parsons et al. 2008; Oliveira et al. 2010). The results presented herein demonstrate, when we expressed individual pup length as a proportion of adult body length, oophagous species are born significantly larger than viviparous lecithotrophic species. In addition, relative pup size at birth was similar among the oophagous species of Lamniformes, the carcharhinid family Pseudotriakidae, and the orectolobiform N. ferrugineus. This result indicates that oophagy confers similar energetic benefits to developing embryos despite arising independently within evolutionarily distinct lineages of sharks (Conrath and Musick 2012; Naylor et al. 2012; Fig. S1) and despite oophagous carcharhinid sharks providing only a finite number of unfertilized ova to each embryo compared to Lamniformes and N. ferrugineus which provide them throughout the gestation period. Herein, we also provide updated quantitative support that oophagy favors fewer offspring facilitated via increased nutritional investment toward a relatively small number of when compared to both placentally viviparous and lecithotrophic viviparous strategies (Wourms 1977; Gilmore 1993; Gilmore et al. 2005; Parsons et al. 2008; Table 3b). Interestingly, when maternal investment is expressed in terms of whole litter mass, but not length, as a proportion of female mass, the distinction between oophagous and lecithotrophic species is neutralized. This suggests that total energetic investment per litter is ultimately similar between reproductive modes.
Differences in relative size at birth as a function of maternal body size has been previously detected in placentally viviparous species from the family Carcharhinidae, in which smaller species had offspring that were 40–60% of maternal body length and larger species had pups that were 20–40% of maternal length (Parsons et al. 2008), contrasting the positive relationship between female body length and fecundity. Our results support the conclusion that the interaction between female length and litter size is a driver of relative pup length for non-oophagous species. However, maximum litter size as a function of maximum female length was a poor predictor of relative pup length for oophagous species, which have a weaker relationship between female body length and litter size. For example, although we identified that oophagous species with smaller bodies had lower fecundity and greater relative pup length at birth compared to large-bodied oophagous species, we found several exceptions. Large-bodied (≥ 4.5 m) oophagous Lamniformes species such as C. carcharias have relatively large litters compared to some other members of their order, but other large-bodied species such as O. ferox, and C. maximus have reduced fecundity relative to their size, but similar relative pup lengths (C. carcharias: mean 0.21 vs. O. ferox and C. maximus: range 0.15–0.22%). In contrast, the comparatively smaller (2.9–3.8 m) I. oxyrinchus has the largest litter sizes observed within the order (i.e., more pups per meter of female), yet relative pup length for this species is also similar (c. 0.19–0.24). This suggests that there is not a trade-off between uterine space and relative pup length in oophagous sharks and pup length is likely to be modulated by different factors.
It is possible that in some cases, reduced fecundity in oophagous species may be compensated for by increased reproductive frequency. Oophagous Lamniformes have a broad range of gestation periods (from 9 months up to 25 months) and temporal patterns of pregnancy also vary among oophagous sharks (i.e., combined gestation and resting period; Gilmore et al. 2005; Castro 2009). For example, highly fecund I. oxyrinchus in the eastern Pacific are thought to have a three-year reproductive cycle comprising a lengthy gestation period of up to 23–25 months and a one-year period of rest (Joung and Hsu 2005). In contrast, Alopiids such as the common thresher Alopias vulpinus have small litters of typically 2–4 pups per reproductive cycle but may have gestation periods as short as 9–12 months (e.g., Gubanov 1972; Natanson and Gervelis 2013) with a one-year rest. Other threshers, such as A. superciliosus and A. pelagicus, forgo reproductive seasonality entirely, with females possibly becoming pregnant again immediately after parturition (Chen et al. 1997; Liu et al. 1999; Das 2016) potentially as an adaptation to maximize lifetime reproductive output for these species. When the maximum litter sizes of these sharks are compared, their average annual fecundity (maximum litter size/reproductive periodicity) is similar, with A. vulpinus, A. pelagicus, and A. superciliosus giving birth to an estimated two pups per year and I. oxyrinchus producing an average of 3.7 pups per year (Appendix 4). However, differing patterns of reproductive periodicity may also exist within single species; for example, populations of C. taurus residing in the northwest Atlantic have been observed to reproduce annually (litters of two pups per year; Gilmore 1993), whereas evidence of a biennial reproductive cycle (average of one pup per year) for this species has been collected in the southwest Atlantic (Lucifora et al. 2002), southeast Atlantic (Dicken 2006), and southwest Pacific (Bansemer and Bennett 2009), suggesting that variability in reproductive periodicity may be influenced by regional adaptation.
Ecological effects on pup length at birth
Our analyses provide evidence that there may be an influence of habitat in the trade-off between litter size and embryonic nourishment during pregnancy. For example, neritic habitats were associated with greater pup length compared to viviparous shark species that had smaller pups in oceanic environments. Larger pups may have a competitive advantage in more sheltered, eutrophic coastal environments than sharks born in the open ocean (e.g., Gilmore et al. 2005). In addition, it is also likely that our model was influenced by the relative abundance of small-bodied placentally viviparous sharks that occupy coastal environments (Castro 1993) which have larger pups relative to their maximum body length than larger-bodied, oceanic species.
Conservation implications
Globally, shark populations are in decline (Baum et al. 2003; Davidson et al. 2016; Dulvy et al. 2021), largely due to ineffective protective measures (Robbins et al. 2006), unsustainable shark-targeted fisheries (de Mitcheson et al. 2018), and mortality through incidental bycatch (Campana et al. 2009). Understanding reproductive potential is an essential component to developing effective conservation strategies with realistic recovery goals (Wheeler et al. 2020). Although some reproductive variables, such as fecundity, pup size, and size at maturity, are easily quantified through direct observation, other measures such as reproductive periodicity and gestation period are more difficult to ascertain; for many species these values are simply unknown. Moreover, our current knowledge of the reproductive habits of sharks is primarily assessed via bycatch monitoring (e.g., Joung and Chen 1995; Beerkircher et al. 2002; Pajuelo et al. 2018), shark fisheries and derbies (e.g., Campana et al. 2005, White 2007), and opportunistic sampling of pregnant females or neonates (e.g., Joung et al. 1996; Cliff et al. 1990) which may limit access to reproductive information about more protected or elusive species. Encouragingly, novel research focusing on non-lethal sampling technology such as steroid hormone analysis (e.g., Awruch et al. 2014; Fujinami et al. 2017; Verkamp et al. 2022), and ultrasonography (e.g., Anderson 2018; Sulikowski et al. 2016) may facilitate improved estimates of these measures without the need for removal of reproductive animals from the population.
Conclusion
Our study supports the hypothesis that placentally viviparous carcharhinids maximize their reproductive fitness by allocating nutrients to larger litters of pups, whereas oophagous species have achieved success in their environments by using the opposite approach, and both forms of matrotrophic viviparity resulted in larger pups on average than lecithotrophic viviparity. As additional life history data become available for viviparous sharks, the differences between reproductive strategies should be examined at a higher resolution such as incorporating as fine-scale habitat use (e.g., temperature, latitude). Future research should continue to elucidate how sharks select and use nursery habitats and how reproductive periodicity varies with environmental features.
Availability of data
All data used in our analyses are made available within our appendices.
Availability of coding
References
Adams KR, Fetterplace LC, Davis AR, Taylor MD, Knott NA (2018) Sharks, rays and abortion: the prevalence of capture-induced parturition in elasmobranchs. Biol Conserv 217:11–27. https://doi.org/10.1016/j.biocon.2017.10.010
Anderson B, Belcher C, Slack J, Gelsleichter J (2018) Evaluation of the use of portable ultrasonography to determine pregnancy status and fecundity in bonnethead shark Sphyrna tiburo. J Fish Biol 93(6):1163–1170. https://doi.org/10.1111/jfb.13831
Awruch, CA, Jones SM, Asorey MG, Barnett A (2014) Non-lethal assessment of the reproductive status of broadnose sevengill sharks (Notorynchus cepedianus) to determine the significance of habitat use in coastal areas. Conserv Physiol 2:cou013. https://doi.org/10.1093/conphys/cou013
Bansemer CS, Bennett MB (2009) Reproductive periodicity, localised movements and behavioural segregation of pregnant Carcharius taurus at Wolf Rock, southeast Queensland. Australia Mar Ecol Prog Ser 374:215–227. https://doi.org/10.3354/meps07741
Baum JK, Myers RA, Kehler DG, Worm B, Harley SJ, Doherty PA (2003) Collapse and conservation of shark populations in the Northwest Atlantic. Science 299:389–392. https://doi.org/10.1126/science.1079777
Beerkircher LR, Cortés E, Shivji M (2002) Characteristics of shark bycatch observed on pelagic longlines off the southeastern United States, 1992–2000. Mar Fish Rev 64:40–49
Buddle AL, Van Dyke JU, Thompson MB, Simpfendorfer CA, Whittington CM (2019) Evolution of placentotrophy: using viviparous sharks as a model to understand vertebrate placental evolution. Mar Freshwat Res 70:908–924. https://doi.org/10.1071/MF18076
Buddle AL, Van Dyke JU, Thompson MB, Simpfendorfer CA, Murphy CR, Dowland SN, Whittington CM (2021) Structure of the paraplacenta and the yolk sac placenta of the viviparous Australian sharpnose shark, Rhizoprionodon taylori. Placenta 108:11–22. https://doi.org/10.1016/j.placenta.2021.03.010
Campana SE, Marks L, Joyce W, Kohler N (2005) Catch, by-catch and indices of population status of blue shark (Prionace glauca) in the Canadian Atlantic. Col Vol Sci Pap ICCAT 58:891–934
Campana SE, Joyce W, Manning MJ (2009) Bycatch and discard mortality in commercially caught blue sharks Prionace glauca assessed using archival satellite pop-up tags. Mar Ecol Prog Ser 387:241–253. https://doi.org/10.3354/meps08109
Castro JI (1993) The shark nursery of Bulls Bay, South Carolina, with a review of the shark nurseries of the southeastern coast of the United States. Environ Biol Fish 38:37–48. https://doi.org/10.1007/BF00842902
Castro JI (2013) A primer on shark reproduction for aquarists. In: Sato K (ed) Reproduction of marine life, birth of new life! Investigating the mysteries of reproduction. Okinawa, Japan: Okinawa Churashima Foundation, pp 52–69
Castro JI, Sato K, Bodine AB (2016) A novel mode of embryonic nutrition in the tiger shark, Galeocerdo cuvier. Mar Biol Res 12:200–205. https://doi.org/10.1080/17451000.2015.1099677
Chen C-T, Liu K-M, Chang Y-C (1997) Reproductive biology of the bigeye thresher shark, Alopias superciliosus (Lowe, 1839) (Chrondrichthyes: Alopiidae), in the northwestern Pacific. Ichthyol Res 44:227–235
Cliff G, Dudley SFJ, Davis B (1990) Sharks caught in the protective gill nets off Natal, South Africa. 3. The shortfin mako shark Isurus oxyrinchus (Rafinesque). S Afr J Marine Sci 9:115–126
Compagno LJ (1977) Phyletic relationships of living sharks and rays. Am Zool 17:303–322
Compagno LJ (1984) FAO species catalogue. Vol. 4. Sharks of the world. An annotated and illustrated catalogue of shark species known to date. FAO Fisheries Synopsis. Food and Agriculture Organization of the United Nations, Rome
Conrath CL, Musick JA (2012) Reproductive biology of elasmobranchs. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives, 2nd edn. CRC Press, Boca Raton, FL, pp 291–306
Cortés E (2000) Life history patterns and correlations in sharks. Rev Fish Sci 8:299–344. https://doi.org/10.1080/10408340308951115
Croux C, Dehon C (2010) Influence functions of the Spearman and Kendall correlation measures. Stat Method Appl 19:497–515. https://doi.org/10.1007/s10260-010-0142-z
Das P (2016) A Report on the Recruitments of Alopias Pelagicus and Alopias Superciliosus in the Andaman Sea. J Aquac Mar Biol 4:11–12. https://doi.org/10.15406/jamb.2016.04.00099
Davidson LN, Krawchuk MA, Dulvy NK (2016) Why have global shark and ray landings declined: improved management or overfishing? Fish Fish 17:438–458. https://doi.org/10.1111/faf.12119
de Carvalho MR (1996) Higher-level elasmobranch phylogeny, basal squaleans, and paraphyly. In: Stiassny MLJ, Parenti LR, Johnson GD (eds) Interrelationships of fishes. Academic Press, San Diego, CA, pp 35–62
de Mitcheson YS, Andersson AA, Hofford A, Law CSW, Hau LCY, Pauly D (2018) Out of control means off the menu: the case for ceasing consumption of luxury products from highly vulnerable species when international trade cannot be adequately controlled; shark fin as a case study. Mar Policy 98:115–120. https://doi.org/10.1016/j.marpol.2018.08.012
Dicken ML (2006) Population dynamics of the raggedtooth shark (Carcharias taurus) along the east coast of South Africa. Dissertation, Rhodes University
Dulvy NK, Forrest RE (2010) Life histories, population dynamics and extinction risks in chondrichthyans. In: Carrier JC, Musick JA, Heithaus MR (eds) Sharks and their relatives II: biodiversity, adaptive physiology, and conservation. CRC Press, Boca Raton, FL, pp 639–679
Dulvy NK, Pacoureau N, Rigby CL et al (2021) Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr Biol 31:4773–4787. https://doi.org/10.1016/j.cub.2021.08.062
Froese R, Pauly D (2016) FishBase. www.fishbase.org. Accessed December 2018
Fujinami Y, Semba Y, Okamoto H, Ohshimo S, Tanaka S (2017) Reproductive biology of the blue shark (Prionace glauca) in the western North Pacific Ocean. Mar Freshwater Res 68:2018–2027. https://doi.org/10.1071/MF16101
Gilmore RG (1993) Reproductive biology of lamnoid sharks. Environ Biol Fish 38:95–114
Gilmore R, Putz O, Dodrill JW (2005) Oophagy, intrauterine cannibalism and reproductive strategy in lamnoid sharks. In: Hamlett WC (ed) Reproductive biology and phylogeny of Chondrichthyes: sharks, batoids and chimaeras. Science Publishers, Enfield, pp 435–462
Gubanov YP (1972) On the biology of the thresher shark Alopias vulpinus (Bonnaterre) in the northwest Indian Ocean. J Ichthyol 12:591–596
Hamlett WC (1989) Evolution and morphogenesis of the placenta in sharks. J Exp Zool 252:35–52
Hamlett WC, Kormanik G, Storrie M, Stevens B, Walker TI (2005) Chondrichthyan parity, lecithotrophy and matrotrophy. In: Hamlett WC (ed) Reproductive biology and phylogeny of Chondrichthyes: sharks, batoids and chimaeras. Science Publishers, Enfield, NH, pp 395–434
Hooton JWL (1991) Randomization tests: statistics for experimenters. Comput Meth Prog Bio 35:43–51. https://doi.org/10.1016/0169-2607(91)90103-Z
Huveneers C, Otway NM, Harcourt RG, Ellis M (2011) Quantification of the maternal-embryonal nutritional relationship of elasmobranchs: case study of wobbegong sharks (genus Orectolobus). J Fish Biol 78:1375–1389. https://doi.org/10.1111/j.1095-8649.2011.02938.x
IUCN (2021) IUCN Red list of threatened species. Version 2021–3. International Union for Conservation of Nature, Gland, Switzerland
Joung S-J, Chen C-T (1995) Reproduction in the sandbar shark, Carcharhinus plumbeus, in the waters off northeastern Taiwan. Copeia 1995:659–665
Joung S-J, Hsu H-H (2005) Reproduction and embryonic development of the shortfin mako, Isurus oxyrinchus Rafinesque, 1810, in the northwestern Pacific. Zool Stud 44:487–496
Joung S-J, Chen C-T, Clark E, Uchida S, Huang WYP (1996) The whale shark, Rhincodon typus, is a livebearer: 300 embryos found in one ‘megamamma’ supreme. Environ Biol Fish 46:219–223
Liu K-M, Chen C-T, Liao T-H, Joung S-J (1999) Age, growth, and reproduction of the pelagic thresher shark, Alopias Pelagicus in the Northwestern Pacific. Copeia 1:68–74
Lucifora LO, Menni RC, Escalante AH (2002) Reproductive ecology and abundance of the sand tiger shark, Carcharias taurus, from the southwestern Atlantic. ICES J Mar Sci 59:553–561. https://doi.org/10.1006/jmsc.2002.1183
Musick JA, Ellis JK (2005) Reproductive evolution of chondrichthyans. In: Hamlett WC (ed) Reproductive biology and phylogeny of Chondrichthyes: sharks, batoids and chimaeras. CRC Press, Boca Raton, FL, pp 45–80
Natanson LJ, Gervelis BJ (2013) The reproductive biology of the common thresher shark in the Western North Atlantic Ocean. Trans Am Fish Soc 142(6):1546–1562. https://doi.org/10.1080/00028487.2013.811099
Naylor GJ, Caira JN, Jensen K, Rosana KA, Straube N, Lakner C (2012) Elasmobranch phylogeny: a mitochondrial estimate based on 595 species. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives, 2nd edn. CRC Press, Boca Raton, FL, pp 31–56
Oliveira P, Hazin FHV, Carvalho F, Rego M, Coelho R, Piercy A, Burgess G (2010) Reproductive biology of the crocodile shark Pseudocarcharias kamoharai. J Fish Biol 76:1655–1670. https://doi.org/10.1111/j.1095-8649.2010.02606.x
Ostrovsky AN, Lidgard S, Gordon DP, Schwaha T, Genikhovich G, Ereskovsky AV (2016) Matrotrophy and placentation in invertebrates: a new paradigm. Biol Rev 91:673–711. https://doi.org/10.1111/brv.12189
Pajuelo M, Alfaro-Shigueto J, Romero M, Pásara-Polack A, Solano A, Vela G, Sarmiento D, Mangel JC (2018) Occurrence and bycatch of juvenile and neonate whale sharks (Rhincodon typus) in Peruvian waters. Pac Sci 72:463–473. https://doi.org/10.2984/72.4.6
Parsons GR, Hoffmayer ER, Hendon JM, Bet-Sayad WV (2008) A review of shark reproductive ecology: life history and evolutionary implications. In: Rocha MJ, Arukwe A, Kapoor BG (eds) Fish reproduction. Science Publishers, Enfield, pp 435–469
Phipson B, Smyth GK (2010) Permutation p-values should never be zero: calculating exact p-values when permutations are randomly drawn. Stat Appl Genet Mol Biol 9:1–12
R Core Development Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available from http://www.R-project.org.
Rizopoulos D (2009) bootStepAIC: bootstrap stepAIC. In R package version 1.2–0 https://cran.r-project.org/web/packages/bootStepAIC/
Robbins WD, Hisano M, Connolly SR, Choat JH (2006) Ongoing collapse of coral-reef shark populations. Curr Biol 16:2314–2319. https://doi.org/10.1016/j.cub.2006.09.044
Sato K, Nakamura M, Tomita T, Toda M, Miyamoto K, Nozu R (2016) How great white sharks nourish their embryos to a large size: evidence of lipid histotrophy in lamnoid shark reproduction. Biol Open 5:1211–1215. https://doi.org/10.1242/bio.017939
Sulikowski JA, Wheeler CR, Gallagher AJ, Prohaska BK, Langan JA, Hammerschlag N (2016) Seasonal and life-stage variation in the reproductive ecology of a marine apex predator the tiger shark Galeocerdo cuvier at a protected female-dominated site. Aquat Biol 24(3):175–184. https://doi.org/10.3354/ab00648
Teshima K, Toda M, Kamei Y, Uchida S, Tamaki M (1997) Reproductive mode of the tawny nurse shark, Nebrius ferrugineus (Elasmobranchii: Ginglymostomatidae) in Okinawa waters, with comments on individuals lacking the second dorsal fin. In: Séret B, Sire J-Y (eds) Proc. of the 5th Indo-Pacific Fisher Conference, Nouméa, New Caledonia, pp. 329–333
Tomita T, Murakumo K, Ueda K, Ashida H, Furuyama R (2019) Locomotion is not a privilege after birth: ultrasound images of viviparous shark embryos swimming from one uterus to the other. Ethology 125:122–126. https://doi.org/10.1111/eth.12828
Verkamp HJ, Hammerschlag N, Quinlan J, Langan JA, Sulikowski JA, Simpfendorfer C (2022) Preliminary investigation of reproductive hormone profiles in the blacktip shark (Carcharhinus limbatus), a placental viviparous species, in southern Florida. Mar Freshw Res 73(4):520–527. https://doi.org/10.1071/MF21235
Weigmann S (2016) Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. J Fish Biol 88:837–1037. https://doi.org/10.1111/jfb.12874
Wheeler CR, Gervais CR, Johnson MS et al (2020) Anthropogenic stressors influence reproduction and development in elasmobranch fishes. Rev Fish Biol Fisher 30:373–386. https://doi.org/10.1007/s11160-020-09604-0
White WT (2007) Biological observations on lamnoid sharks (Lamniformes) caught by fisheries in eastern Indonesia. J Mar Biol Assoc UK 87:781–788. https://doi.org/10.1017/S0025315407056391
Williams GC (1966) Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am Nat 100:687–690
Wourms JP (1977) Reproduction and development in chondrichthyan fishes. Am Zool 17:379–410
Wourms JP (1981) Viviparity–the maternal-fetal relationship in fishes. Am Zool 21:473–515
Wourms JP, Lombardi J (1992) Reflections on the evolution of piscine viviparity. Am Zool 32:276–293
Yano K, Musick JA (1992) Comparison of morphometrics of Atlantic and Pacific specimens of the false catshark, Pseudotriakis microdon, with notes on stomach contents. Copeia 1992:877–886
Acknowledgements
We thank A. Nazari and J. Smith for their assistance in data collection during early project development. We thank B. Quinn for their feedback on previous drafts, and the anonymous reviewers whose comments strengthened this manuscript.
Funding
Open access funding provided by Fisheries & Oceans Canada. No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceived project: EM. Analyzed data: CNW, EM. Original draft preparation: EM, CNW. Review and editing: EM, CNW, JS.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no conflicts of interest to declare for this project.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miller, E., Wails, C.N. & Sulikowski, J. It’s a shark-eat-shark world, but does that make for bigger pups? A comparison between oophagous and non-oophagous viviparous sharks. Rev Fish Biol Fisheries 32, 1019–1033 (2022). https://doi.org/10.1007/s11160-022-09707-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-022-09707-w