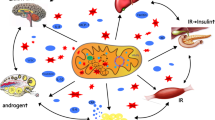
Abstract
Mitochondrial DNA (mtDNA) epigenetic modifications have recently gained attention in a plethora of complex diseases, including polycystic ovary syndrome (PCOS), a common cause of infertility in women of reproductive age. Herein we discussed mtDNA epigenetic modifications and their impact on nuclear-mitochondrial interactions in general and the latest advances indicating the role of mtDNA methylation in the pathophysiology of PCOS. We highlighted epigenetic changes in nuclear-related mitochondrial genes, including nuclear transcription factors that regulate mitochondrial function and may be involved in the development of PCOS or its related traits. Additionally, therapies targeting mitochondrial epigenetics, including time-restricted eating (TRE), which has been shown to have beneficial effects by improving mitochondrial function and may be mediated by epigenetic modifications, have also been discussed. As PCOS has become a major metabolic disorder and a risk factor for obesity, cardiometabolic disorders, and diabetes, lifestyle/behavior intervention using TRE that reinforces feeding-fasting rhythms without reducing caloric intake may be a promising therapeutic strategy for attenuating the pathogenesis. Furthermore, future perspectives in the area of mitochondrial epigenetics are described.

Similar content being viewed by others
Abbreviations
- 5-hmC:
-
5 Hydroxymethylcytosine
- 5-mC:
-
5-methylcytosine
- 5-MTHF:
-
5-methyl tetrahydrofolate
- αKG:
-
Alpha-ketoglutarate
- AMP:
-
Adenosine Monophosphate
- AMPK:
-
AMP-activated protein kinase
- ATP:
-
Adenosine Triphosphate
- BHMT:
-
Betaine homocysteine methyltransferase
- D-loop:
-
Displacement loop
- DNMT:
-
DNA methyltransferase
- DNMT1:
-
DNA methyltransferase 1
- DNMT3:
-
DNA methyltransferase 3
- D NMT3A:
-
DNA methyltransferase 3 alpha
- DNMT3B:
-
DNA methyltransferase 3 Beta
- DNMT3L:
-
DNA methyltransferase 3 Like
- ETC:
-
Electron transport chain
- GNMT:
-
Glycine N-methyltransferase
- Hcy:
-
Homocysteine
- IVF:
-
In-vitro fertilization
- MBE:
-
Mitochondrial bifunctional enzyme
- Met:
-
Methionine
- miRNA:
-
micro-RNAs
- MS:
-
Methionine synthase
- mtDNA:
-
Mitochondrial DNA
- mTERFs:
-
Mitochondrial transcription termination factors
- MTHF:
-
Methyl tetrahydrofolate
- MTS:
-
Mitochondrial targeting sequence
- nDNA:
-
Nuclear DNA
- ncRNA:
-
non-coding RNAs
- NRF-1:
-
Nuclear respiratory factor 1
- NRF-2:
-
Nuclear respiratory factor 2
- OXPHOS:
-
Oxidative Phosphorylation
- PCOS:
-
Polycystic ovary syndrome
- PGC-1α:
-
Peroxisome proliferator-activated receptor-gamma coactivator
- PGT-A:
-
Preimplantation genetic testing for aneuploidies
- POLRMT:
-
Mitochondrial RNA polymerase
- PPAR:
-
Peroxisome proliferator-activated receptors
- PPARG:
-
Peroxisome Proliferator-Activated Receptor Gamma
- PPARGC1A:
-
Peroxisome Proliferator-activated Receptor Gamma Coactivator-1 Alpha
- PPARGC1B:
-
Peroxisome Proliferator-activated Receptor Gamma Coactivator-1 Beta
- PPARD:
-
Peroxisome Proliferator-Activated Receptor Delta
- ROS:
-
Reactive Oxygen Species
- rRNA:
-
Ribosomal ribonucleic acid
- SAM:
-
S-adenosylmethionine
- SAMC:
-
SAM carrier
- SIRTs:
-
Sirtuins
- TCA:
-
Tricarboxylic acid cycle
- TET:
-
Ten-eleven translocation
- TFAM:
-
Mitochondrial transcription Factor A
- TFB2M:
-
Mitochondrial transcription Factor B2
- THF:
-
Tetrahydrofolate
- TRE:
-
Time-restricted eating
- tRNA:
-
Transfer ribonucleic acid
References
Duarte FV, Amorim JA, Palmeira CM, Rolo AP. Regulation of mitochondrial function and its impact in metabolic stress. Curr Med Chem. 2015;22:2468–79.
Shukla P, Mukherjee S. Mitochondrial dysfunction: an emerging link in the pathophysiology of polycystic ovary syndrome. Mitochondrion. 2020;52:24–39.
Shukla P, Mukherjee S, Patil A. Identification of variants in mitochondrial D-Loop and OriL region and analysis of mitochondrial DNA copy number in women with polycystic ovary syndrome. DNA Cell Biol. 2020;39:1458–66.
Sharma N, Pasala MS, Prakash A, Mitochondrial DNA. Epigenetics and environment. Environ Mol Mutagen. 2019;60:668–82.
Wang Y, Bogenhagen DF. Human mitochondrial DNA nucleoids are linked to protein folding machinery and metabolic enzymes at the mitochondrial inner membrane. J Biol Chem. 2006;281:25791–802.
Walker BR, Moraes CT. Nuclear-mitochondrial interactions. Biomolecules. 2022;12:427.
Patil V, Cuenin C, Chung F, Aguilera JRR, Fernandez-Jimenez N, Romero-Garmendia I, et al. Human mitochondrial DNA is extensively methylated in a non-CpG context. Nucleic Acids Res. 2019;47:10072–85.
Ghosh S, Sengupta S, Scaria V. Comparative analysis of human mitochondrial methylomes shows distinct patterns of epigenetic regulation in mitochondria. Mitochondrion. 2014;18:58–62.
van der Wijst MG, Rots MG. Mitochondrial epigenetics: an overlooked layer of regulation? Trends Genet TIG. 2015;31:353–6.
Shock LS, Thakkar PV, Peterson EJ, Moran RG, Taylor SM. DNA methyltransferase 1, cytosine methylation, and cytosine hydroxymethylation in mammalian mitochondria. Proc Natl Acad Sci USA. 2011;108:3630–5.
Castegna A, Iacobazzi V, Infantino V. The mitochondrial side of epigenetics. Physiol Genom. 2015;47:299–307.
Bellizzi D, D’Aquila P, Scafone T, Giordano M, Riso V, Riccio A, et al. The control region of mitochondrial DNA shows an unusual CpG and non-CpG methylation pattern. DNA Res. 2013;20:537–47.
Manev H, Dzitoyeva S. Progress in mitochondrial epigenetics. Biomol Concepts. 2013;4:381–9.
Ghosh S, Sengupta S, Scaria V. Hydroxymethyl cytosine marks in the human mitochondrial genome are dynamic in nature. Mitochondrion. 2016;27:25–31.
Anderson KA, Hirschey MD. Mitochondrial protein acetylation regulates metabolism. Essays Biochem. 2012;52:23–35.
Ducker GS, Rabinowitz JD. One-carbon metabolism in health and disease. Cell Metabol. 2017;25:27–42.
Reina-Campos M, Diaz-Meco MT, Moscat J. The complexity of the serine glycine one-carbon pathway in cancer. J Cell Biol. 2020;219:e201907022.
Agrimi G, Di Noia MA, Marobbio CM, Fiermonte G, Lasorsa FM, Palmieri F. Identification of the human mitochondrial S-adenosylmethionine transporter: bacterial expression, reconstitution, functional characterization and tissue distribution. Biochem J. 2004;379:183–90.
Gusic M, Prokisch H, ncRNAs. New players in mitochondrial health and disease? Front Genet. 2020;11:95.
A FCL. Mitochondrial metabolism and DNA methylation: a review of the interaction between two genomes. Clin Epigenetics. 2020;12:182.
Ro S, Ma HY, Park C, Ortogero N, Song R, Hennig GW, et al. The mitochondrial genome encodes abundant small non-coding RNAs. Cell Res. 2013;23:759–74.
Zhu D, Li X, Tian Y. Mitochondrial-to-nuclear communication in aging: an epigenetic perspective. Trends Biochem Sci. 2022;47:645–59.
Guha M, Avadhani NG. Mitochondrial retrograde signaling at the crossroads of tumor bioenergetics, genetics and epigenetics. Mitochondrion. 2013;13:577–91.
Shukla P, Singh KK. Uncovering mitochondrial determinants of racial disparities in ovarian cancer. Trends in cancer. 2021;7:93–7.
Minocherhomji S, Tollefsbol TO, Singh KK. Mitochondrial regulation of epigenetics and its role in human diseases. Epigenetics. 2012;7:326–34.
Smiraglia DJ, Kulawiec M, Bistulfi GL, Gupta SG, Singh KK. A novel role for mitochondria in regulating epigenetic modification in the nucleus. Cancer Biol Ther. 2008;7:1182–90.
Bellizzi D, D’Aquila P, Giordano M, Montesanto A, Passarino G. Global DNA methylation levels are modulated by mitochondrial DNA variants. Epigenomics. 2012;4:17–27.
Kelly RD, Rodda AE, Dickinson A, Mahmud A, Nefzger CM, Lee W, et al. Mitochondrial DNA haplotypes define gene expression patterns in pluripotent and differentiating embryonic stem cells. Stem Cells. 2013;31:703–16.
Wiese M, Bannister AJ. Two genomes, one cell: mitochondrial-nuclear coordination via epigenetic pathways. Mol Metab. 2020;38:100942.
Fan W, Evans R. PPARs and ERRs: molecular mediators of mitochondrial metabolism. Curr Opin Cell Biol. 2015;33:49–54.
Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol. 2005;25:1354–66.
Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93:884s–90.
Lombard DB, Tishkoff DX, Bao J. Mitochondrial sirtuins in the regulation of mitochondrial activity and metabolic adaptation. Handb Exp Pharmacol. 2011;206:163–88.
Cavalcante GC, Magalhães L, Ribeiro-Dos-Santos Â, Vidal AF. Mitochondrial epigenetics: non-coding RNAs as a novel layer of complexity. Int J Mol Sci. 2020;21:1838.
Dennett CC, Simon J. The role of polycystic ovary syndrome in reproductive and metabolic health: overview and approaches for treatment. Diabetes Spectr. 2015;28:116–20.
Toosy S, Sodi R, Pappachan JM. Lean polycystic ovary syndrome (PCOS): an evidence-based practical approach. J Diabetes Metab Disord. 2018;17:277–85.
Kosova G, Urbanek M. Genetics of the polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373:29–38.
Janssen JJE, Grefte S, Keijer J, de Boer VCJ. Mito-nuclear communication by mitochondrial metabolites and its regulation by B-Vitamins. Front Physiol. 2019;10:78.
Babayev E, Seli E. Oocyte mitochondrial function and reproduction. Curr Opin Obst Gynecol. 2015;27:175–81.
Jia L, Li J, He B, Jia Y, Niu Y, Wang C, et al. Abnormally activated one-carbon metabolic pathway is associated with mtDNA hypermethylation and mitochondrial malfunction in the oocytes of polycystic gilt ovaries. Sci Rep. 2016;6:19436.
Jia L, Zeng Y, Hu Y, Liu J, Yin C, Niu Y, et al. Homocysteine impairs porcine oocyte quality via deregulation of one-carbon metabolism and hypermethylation of mitochondrial DNA†. Biol Reprod. 2019;100:907–16.
Zhao H, Zhao Y, Ren Y, Li M, Li T, Li R, et al. Epigenetic regulation of an adverse metabolic phenotype in polycystic ovary syndrome: the impact of the leukocyte methylation of PPARGC1A promoter. Fertil Steril. 2017;107:467–74.e5.
Skov V, Glintborg D, Knudsen S, Jensen T, Kruse TA, Tan Q, et al. Reduced expression of nuclear-encoded genes involved in mitochondrial oxidative metabolism in skeletal muscle of insulin-resistant women with polycystic ovary syndrome. Diabetes. 2007;56:2349–55.
Lambertini L, Saul SR, Copperman AB, Hammerstad SS, Yi Z, Zhang W, et al. Intrauterine reprogramming of the polycystic ovary syndrome: evidence from a pilot study of cord blood global methylation analysis. Front Endocrinol. 2017;8:352.
Kokosar M, Benrick A, Perfilyev A, Fornes R, Nilsson E, Maliqueo M, et al. Epigenetic and transcriptional alterations in human adipose tissue of polycystic ovary syndrome. Sci Rep. 2016;6:22883.
Zhao Y, Fu L, Li R, Wang LN, Yang Y, Liu NN, et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: plasma metabolomics analysis. BMC Med. 2012;10:153.
Rajska A, Buszewska-Forajta M, Rachoń D, Markuszewski MJ. Metabolomic insight into polycystic ovary syndrome-an overview. Int J Mol Sci. 2020;21:4853.
Liu R, Bai S, Zheng S, Zhu X, Zhang Y, Xu B, et al. Identification of the metabolomics signature of human follicular fluid from PCOS women with insulin resistance. Dis Markers. 2022;2022:6877541.
Chen H, Dzitoyeva S, Manev H. Effect of valproic acid on mitochondrial epigenetics. Eur J Pharmacol. 2012;690:51–9.
Chestnut BA, Chang Q, Price A, Lesuisse C, Wong M, Martin LJ. Epigenetic regulation of motor neuron cell death through DNA methylation. J Neurosci. 2011;31:16619–36.
Asemi Z, Karamali M, Esmaillzadeh A. Metabolic response to folate supplementation in overweight women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Mol Nutr Food Res. 2014;58:1465–73.
Gill S, Le HD, Melkani GC, Panda S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science. 2015;347:1265–9.
Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–60.
Longo VD, Panda S, Fasting. Circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23:1048–59.
Melkani GC, Panda S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J Physiol. 2017;595:3691–700.
Villanueva JE, Livelo C, Trujillo AS, Chandran S, Woodworth B, Andrade L, et al. Time-restricted feeding restores muscle function in Drosophila models of obesity and circadian-rhythm disruption. Nat Commun. 2019;10:2700.
Livelo C, Guo Y, Melkani GC. A skeletal muscle-centric view on time-restricted feeding and obesity under various metabolic challenges in humans and animals. Int J Mol Sci. 2022;24:422.
Sardon Puig L, Valera-Alberni M, Cantó C, Pillon NJ. Circadian rhythms and mitochondria: connecting the dots. Front Genet. 2018;9:452.
Li C, Xing C, Zhang J, Zhao H, Shi W, He B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J Transl Med. 2021;19:148.
Chaix A, Manoogian ENC, Melkani GC, Panda S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr. 2019;39:291–315.
Bordoni L, Petracci I, Mlodzik-Czyzewska M, Malinowska AM, Szwengiel A, Sadowski M, et al. Mitochondrial DNA and epigenetics: investigating interactions with the one-carbon metabolism in obesity. Oxidative Med Cell Longev. 2022;2022:9171684.
Konopka AR, Asante A, Lanza IR, Robinson MM, Johnson ML, Dalla Man C, et al. Defects in mitochondrial efficiency and H2O2 emissions in obese women are restored to a lean phenotype with aerobic exercise training. Diabetes. 2015;64:2104–15.
Cui N, Wang H, Wang W, Zhang J, Xu Y, Jiang L et al. Impact of body mass index on outcomes of in vitro fertilization/intracytoplasmic sperm injection among polycystic ovarian syndrome patients. Cell Physiol Biochem. 2016;39:1723–34.
Parikh FR, Athalye AS, Naik NJ, Naik DJ, Sanap RR, Madon PF. Preimplantation genetic testing: its evolution, where are we today? J Hum Reprod Sci. 2018;11:306–14.
Cagnone GL, Tsai TS, Makanji Y, Matthews P, Gould J, Bonkowski MS, et al. Restoration of normal embryogenesis by mitochondrial supplementation in pig oocytes exhibiting mitochondrial DNA deficiency. Sci Rep. 2016;6:23229.
Acknowledgements
The authors acknowledge the necessary support provided by the Indian Council of Medical Research-National Institute for Research in Reproductive and Child Health (ICMR-NIRRCH) (Rev/1297/08-2022).
Author information
Authors and Affiliations
Contributions
Both authors contributed to the writing, critically edited each version of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
NA.
Conflict of interest
The authors declared no financial or non-finacial interest involved with this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shukla, P., Melkani, G.C. Mitochondrial epigenetic modifications and nuclear-mitochondrial communication: A new dimension towards understanding and attenuating the pathogenesis in women with PCOS. Rev Endocr Metab Disord 24, 317–326 (2023). https://doi.org/10.1007/s11154-023-09789-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-023-09789-2