Abstract
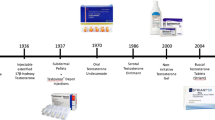
The pursuit of longevity, which during the Renaissance era was limited to longing for miraculous ways of rejuvenation, such as bathing in the fountain of youth, took a scientific turn in 1889 with the publication of Brown-Sequard’s self-experiments with an extract of animal testes, which apparently improved his vitality, physical strength and cognition. This extract, marketed then as the "Elixir of Life", was sold for decades throughout Europe and North America. However, recent replication of Brown-Sequard’s experiments demonstrated that such an extract only contains homeopathic concentrations of testosterone that are insufficient to exert any biological effect. Thus, the birth of Andrology began with a placebo effect. Over the past few decades, the quest for compounds that might lead to rejuvenation has regained traction, with testosterone being at the forefront. Though clinical practice guidelines advocate testosterone therapy in men with organic hypogonadism—the only indication approved by the Food and Drug Administration—testosterone continues to be marketed as a wonder drug with rejuvenating effects on sexual function, vitality, and a host of other unproven benefits. Additionally, the epidemic of obesity and diabetes, conditions associated with low testosterone, has further brought testosterone into the limelight. Although the number of testosterone prescriptions written have increased several-fold in the past two decades, carefully conducted randomized trials suggest modest benefits of testosterone therapy. At the same time, safety concerns, particularly in older men, remain valid.






Similar content being viewed by others
References
Brown-Séquard C. Note on the effects produced on man by subcutaneous injections of a liquid obtained from the testicles of animals. The Lancet. 1889;134(3438):105–7.
Borell M. Organotherapy, british physiology, and discovery of the internal secretions. J Hist Biol. 1976;9(2):235–68.
Cussons AJ, Bhagat CI, Fletcher SJ, Walsh JP. Brown-Sequard revisited: a lesson from history on the placebo effect of androgen treatment. Med J Aust. 2002;177(11–12):678–9.
Vermeulen A, Rubens R, Verdonck L. Testosterone secretion and metabolism in male senescence. J Clin Endocrinol Metab. 1972;34(4):730–5.
Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(5):1715–44.
Veldhuis JD, Keenan DM, Liu PY, Takahashi PY. Kinetics of removal of intravenous testosterone pulses in normal men. Eur J Endocrinol. 2010;162(4):787–94.
Rengachary SS, Colen C, Guthikonda M. Charles-Edouard Brown-Sequard: an eccentric genius. Neurosurgery. 2008;62(4):954–64; discussion 64.
Kanayama G, Pope HG Jr. History and epidemiology of anabolic androgens in athletes and non-athletes. Mol Cell Endocrinol. 2018;464:4–13.
Pope HG Jr, Olivardia R, Gruber A, Borowiecki J. Evolving ideals of male body image as seen through action toys. Int J Eat Disord. 1999;26(1):65–72.
Pope HG Jr, Wood RI, Rogol A, Nyberg F, Bowers L, Bhasin S. Adverse health consequences of performance-enhancing drugs: an Endocrine Society scientific statement. Endocr Rev. 2014;35(3):341–75.
Sagoe D, Molde H, Andreassen CS, Torsheim T, Pallesen S. The global epidemiology of anabolic-androgenic steroid use: a meta-analysis and meta-regression analysis. Ann Epidemiol. 2014;24(5):383–98.
Pope HG Jr, Kanayama G, Athey A, Ryan E, Hudson JI, Baggish A. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: current best estimates. Am J Addict. 2014;23(4):371–7.
Blashill AJ, Safren SA. Sexual orientation and anabolic-androgenic steroids in U.S. adolescent boys. Pediatrics. 2014;133(3):469–75.
de Ronde W, Smit DL. Anabolic androgenic steroid abuse in young males. Endocr Connect. 2020;9(4):R102–11.
Bonnecaze AK, O’Connor T, Aloi JA. Characteristics and attitudes of men using Anabolic Androgenic Steroids (AAS): A survey of 2385 Men. Am J Mens Health. 2020;14(6):1557988320966536.
Mullen C, Whalley BJ, Schifano F, Baker JS. Anabolic androgenic steroid abuse in the United Kingdom: An update. Br J Pharmacol. 2020;177(10):2180–98.
Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173(15):1465–6.
Bandari J, Ayyash OM, Emery SL, Wessel CB, Davies BJ. Marketing and testosterone treatment in the USA: a systematic review. Eur Urol Focus. 2017;3(4–5):395–402.
Handelsman DJ. Global trends in testosterone prescribing, 2000–2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548–51.
Koo K, Yap RL. Trends in urological direct-to-consumer advertising during prime-time television news programs. Urology Practice. 2017;4(1):7–13.
Mintzes B. The marketing of testosterone treatments for age-related low testosterone or “Low T.” Curr Opin Endocrinol Diabetes Obes. 2018;25(3):224–30.
Layton JB, Kim Y, Alexander GC, Emery SL. Association between direct-to-consumer advertising and testosterone testing and initiation in the United States, 2009–2013. JAMA. 2017;317(11):1159–66.
Leonardo Alves T, Martins de Freitas AF, van Eijk ME, Mantel-Teeuwisse AK. Compliance of disease awareness campaigns in printed Dutch media with national and international regulatory guidelines. PLoS One. 2014;9(9):e106599.
Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Baltimore Longitudinal Study of A. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–31.
Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–35.
Cauley JA, Fluharty L, Ellenberg SS, Gill TM, Ensrud KE, Barrett-Connor E, et al. Recruitment and screening for the testosterone trials. J Gerontol A Biol Sci Med Sci. 2015;70(9):1105–11.
Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611–24.
Jasuja GK, Bhasin S, Rose AJ. Patterns of testosterone prescription overuse. Curr Opin Endocrinol Diabetes Obes. 2017;24(3):240–5.
Cunningham GR, Stephens-Shields AJ, Rosen RC, Wang C, Bhasin S, Matsumoto AM, et al. Testosterone treatment and sexual function in older men with low testosterone levels. J Clin Endocrinol Metab. 2016;101(8):3096–104.
Qaseem A, Snow V, Denberg TD, Casey DE Jr, Forciea MA, Owens DK, et al. Hormonal testing and pharmacologic treatment of erectile dysfunction: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2009;151(9):639–49.
Spitzer M, Huang G, Basaria S, Travison TG, Bhasin S. Risks and benefits of testosterone therapy in older men. Nat Rev Endocrinol. 2013;9(7):414–24.
Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, Ellenberg SS, Cauley JA, Ensrud KE, et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: A controlled clinical trial. JAMA Intern Med. 2017;177(4):471–9.
Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Lessons from the testosterone trials. Endocr Rev. 2018;39(3):369–86.
Watts NB, Adler RA, Bilezikian JP, Drake MT, Eastell R, Orwoll ES, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802–22.
Roy CN, Snyder PJ, Stephens-Shields AJ, Artz AS, Bhasin S, Cohen HJ, et al. Association of testosterone levels with anemia in older men: A controlled clinical trial. JAMA Intern Med. 2017;177(4):480–90.
Shahani S, Braga-Basaria M, Maggio M, Basaria S. Androgens and erythropoiesis: past and present. J Endocrinol Invest. 2009;32(8):704–16.
Zitzmann M. Testosterone, mood, behaviour and quality of life. Andrology. 2020;8(6):1598–605.
Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of the “androgen deprivation syndrome” in men receiving androgen deprivation for prostate cancer. Arch Intern Med. 2006;166(4):465–71.
Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–22.
Bhasin S, Apovian CM, Travison TG, Pencina K, Moore LL, Huang G, et al. Effect of protein intake on lean body mass in functionally limited older men: a randomized clinical trial. JAMA Intern Med. 2018;178(4):530–41.
Basaria S, Harman SM, Travison TG, Hodis H, Tsitouras P, Budoff M, et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: a randomized clinical trial. JAMA. 2015;314(6):570–81.
Ellison-Barnes A, Johnson S, Gudzune K. Trends in obesity prevalence among adults aged 18 through 25 years, 1976–2018. JAMA. 2021;326(20):2073–4.
Liu B, Du Y, Wu Y, Snetselaar LG, Wallace RB, Bao W. Trends in obesity and adiposity measures by race or ethnicity among adults in the United States 2011–18: population based study. BMJ. 2021;372: n365.
Goudswaard LJ, Bell JA, Hughes DA, Corbin LJ, Walter K, Davey Smith G, et al. Effects of adiposity on the human plasma proteome: observational and Mendelian randomisation estimates. Int J Obes (Lond). 2021;45(10):2221–9.
Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev. 2017;38(4):302–24.
Dhindsa S, Miller MG, McWhirter CL, Mager DE, Ghanim H, Chaudhuri A, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33(6):1186–92.
Grossmann M, Matsumoto AM. A perspective on middle-aged and older men with functional hypogonadism: focus on holistic management. J Clin Endocrinol Metab. 2017;102(3):1067–75.
Jasuja GK, Bhasin S, Reisman JI, Hanlon JT, Miller DR, Morreale AP, et al. Who gets testosterone? patient characteristics associated with testosterone prescribing in the veteran affairs system: a cross-sectional Study. J Gen Intern Med. 2017;32(3):304–11.
Ng Tang Fui M, Prendergast LA, Dupuis P, Raval M, Strauss BJ, Zajac JD, et al. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Med. 2016;14(1):153.
Mangolim AS, Brito LAR, Nunes-Nogueira VDS. Effectiveness of testosterone replacement in men with obesity: a systematic review and meta-analysis. Eur J Endocrinol. 2021;186(1):123–35.
Ng Tang Fui M, Hoermann R, Zajac JD, Grossmann M. The effects of testosterone on body composition in obese men are not sustained after cessation of testosterone treatment. Clin Endocrinol (Oxf). 2017;87(4):336–43.
Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295(11):1288–99.
Pitteloud N, Mootha VK, Dwyer AA, Hardin M, Lee H, Eriksson KF, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005;28(7):1636–42.
Wittert G, Bracken K, Robledo KP, Grossmann M, Yeap BB, Handelsman DJ, et al. Testosterone treatment to prevent or revert type 2 diabetes in men enrolled in a lifestyle programme (T4DM): a randomised, double-blind, placebo-controlled, 2-year, phase 3b trial. Lancet Diabetes Endocrinol. 2021;9(1):32–45.
Gagliano-Juca T, Basaria S. Testosterone replacement therapy and cardiovascular risk. Nat Rev Cardiol. 2019;16(9):555–74.
Santella C, Renoux C, Yin H, Yu OHY, Azoulay L. Testosterone replacement therapy and the risk of prostate cancer in men with late-onset hypogonadism. Am J Epidemiol. 2019;188(9):1666–73.
Loeb S, Folkvaljon Y, Damber JE, Alukal J, Lambe M, Stattin P. Testosterone replacement therapy and risk of favorable and aggressive prostate cancer. J Clin Oncol. 2017;35(13):1430–6.
Walsh TJ, Shores MM, Krakauer CA, Forsberg CW, Fox AE, Moore KP, et al. Testosterone treatment and the risk of aggressive prostate cancer in men with low testosterone levels. PLoS ONE. 2018;13(6): e0199194.
Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS ONE. 2014;9(1): e85805.
Vigen R, O’Donnell CI, Baron AE, Grunwald GK, Maddox TM, Bradley SM, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829–36.
Martinez C, Suissa S, Rietbrock S, Katholing A, Freedman B, Cohen AT, et al. Testosterone treatment and risk of venous thromboembolism: population based case-control study. BMJ. 2016;355: i5968.
Food and Drug Administration (FDA). Center for drug evaluation and research. Minutes of the Joint Meeting of the Bone, Reproductive and Urologic Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. 2014.
Budoff MJ, Ellenberg SS, Lewis CE, Mohler ER 3rd, Wenger NK, Bhasin S, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317(7):708–16.
Gagliano-Juca T, Basaria S. Trials of testosterone replacement reporting cardiovascular adverse events. Asian J Androl. 2018;20(2):131–7.
Li H, Benoit K, Wang W, Motsko S. Association between use of exogenous testosterone therapy and risk of venous thrombotic events among exogenous testosterone treated and untreated men with hypogonadism. J Urol. 2016;195(4 Pt 1):1065–72.
Sharma R, Oni OA, Chen G, Sharma M, Dawn B, Sharma R, et al. Association between testosterone replacement therapy and the incidence of DVT and pulmonary embolism: a retrospective cohort study of the veterans administration database. Chest. 2016;150(3):563–71.
Shores MM, Walsh TJ, Korpak A, Krakauer C, Forsberg CW, Fox AE, et al. Association between testosterone treatment and risk of incident cardiovascular events among us male veterans with low testosterone levels and multiple medical comorbidities. J Am Heart Assoc. 2021;10(17): e020562.
Baillargeon J, Urban RJ, Kuo YF, Ottenbacher KJ, Raji MA, Du F, et al. Risk of myocardial infarction in older men receiving testosterone therapy. Ann Pharmacother. 2014;48(9):1138–44.
Sharma R, Oni OA, Gupta K, Chen G, Sharma M, Dawn B, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36(40):2706–15.
Anderson JL, May HT, Lappe DL, Bair T, Le V, Carlquist JF, et al. Impact of testosterone replacement therapy on myocardial infarction, stroke, and death in men with low testosterone concentrations in an Integrated Health Care System. Am J Cardiol. 2016;117(5):794–9.
Bhasin S, Lincoff AM, Basaria S, Bauer DC, Boden WE, Cunningham GR, et al. Effects of long-term testosterone treatment on cardiovascular outcomes in men with hypogonadism: Rationale and design of the TRAVERSE study. Am Heart J. 2022;245:41–50.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
No sources of funding, financial or non-financial interests are declared. Due to the nature of the article (review) no study-specific approval by the appropriate ethics committee for research involving humans and/or animals, neither informed consent if the research involved human participants, and a statement on welfare of animals if the research involved animals is provided.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gagliano-Jucá, T., Alvarez, M. & Basaria, S. The medicalization of testosterone: reinventing the elixir of life. Rev Endocr Metab Disord 23, 1275–1284 (2022). https://doi.org/10.1007/s11154-022-09751-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-022-09751-8