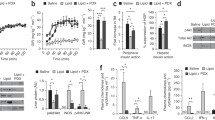
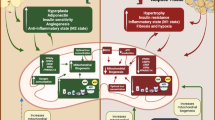
Abstract
Insulin resistance and metabolic dysfunction in skeletal muscle play a major role in the development of the metabolic syndrome and type 2 diabetes. Numerous mechanisms have been proposed to explain the pathophysiology of obesity-linked metabolic dysfunction and this review will focus on the contributing role of adiponectin and inflammation. The beneficial effects of adiponectin on both insulin action and inflammation are now well documented and will be reviewed. More recent work provided new insights into adiponectin signaling mechanisms. The development of strategies to mimic adiponectin action holds promise that adiponectin-based compounds may translate into effective therapeutic applications. We will also discussed the novel role of long chain ω-3 PUFA-derived resolution mediators, which in addition to resolving inflammation, can also exert glucoregulatory effects in models of obesity and insulin resistance. We will focus on one resolution mediator, protectin DX (PDX), which was recently shown to act as a muscle interleukin-6 secretagogue. PDX and its isomer PD1 also enhance adiponectin expression and action. Ultimately, it is via a better understanding the molecular mechanisms of action via which inflammation, insulin resistance and metabolic dysfunction occur in skeletal muscle, and also how they crosstalk with each other, that we can generate new and improved therapies for obesity-linked metabolic complications.


Similar content being viewed by others
References
Sherwin R, Jastreboff AM. Year in diabetes 2012: The diabetes tsunami. J Clin Endocrinol Metab. 2012;97(12):4293–301. doi:10.1210/jc.2012-3487.
Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010;2010:476279. doi:10.1155/2010/476279.
Raschke S, Eckel J. Adipo-myokines: Two sides of the same coin–mediators of inflammation and mediators of exercise. Mediat Inflamm. 2013;2013:320724. doi:10.1155/2013/320724.
Henriksen T, Green C, Pedersen BK. Myokines in myogenesis and health. Recent Patents Biotechnol. 2012;6(3):167–71.
Xu E, Schwab M, Marette A. Role of protein tyrosine phosphatases in the modulation of insulin signaling and their implication in the pathogenesis of obesity-linked insulin resistance. Rev Endocr Metab Disord. 2014;15(1):79–97. doi:10.1007/s11154-013-9282-4.
Fan W, Atkins AR, Yu RT, Downes M, Evans RM. Road to exercise mimetics: Targeting nuclear receptors in skeletal muscle. J Mol Endocrinol. 2013;51(3):T87–T100. doi:10.1530/JME-13-0258.
Liu Y, Sweeney G. Adiponectin action in skeletal muscle. Best Pract Res Clin Endocrinol Metab. 2014;28(1):33–41. doi:10.1016/j.beem.2013.08.003.
Scheid MP, Sweeney G. The role of adiponectin signaling in metabolic syndrome and cancer. Rev Endocr Metab Disord. 2014;15(2):157–67. doi:10.1007/s11154-013-9265-5.
Liu Y, Retnakaran R, Hanley A, Tungtrongchitr R, Shaw C, Sweeney G. Total and high molecular weight but not trimeric or hexameric forms of adiponectin correlate with markers of the metabolic syndrome and liver injury in Thai subjects. J Clin Endocrinol Metab. 2007;92(11):4313–8. doi:10.1210/jc.2007-0890.
Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab. 2013;2(3):133–41. doi:10.1016/j.molmet.2013.04.001.
Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17(2):185–96. doi:10.1016/j.cmet.2013.01.001.
Kuoppamaa H, Skrobuk P, Sihvo M, Hiukka A, Chibalin AV, Zierath JR, et al. Globular adiponectin stimulates glucose transport in type 2 diabetic muscle. Diabetes Metab Res Rev. 2008;24(7):554–62. doi:10.1002/dmrr.883.
Cheng KK, Lam KS, Wang B, Xu A. Signaling mechanisms underlying the insulin-sensitizing effects of adiponectin. Best Pract Res Clin Endocrinol Metab. 2014;28(1):3–13. doi:10.1016/j.beem.2013.06.006.
Liu Y, Turdi S, Park T, Morris NJ, Deshaies Y, Xu A, et al. Adiponectin corrects high-fat diet-induced disturbances in muscle metabolomic profile and whole-body glucose homeostasis. Diabetes. 2013;62(3):743–52. doi:10.2337/db12-0687.
Combs TP, Marliss EB. Adiponectin signaling in the liver. Rev Endocr Metab Disord. 2014;15(2):137–47. doi:10.1007/s11154-013-9280-6.
Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T. Adiponectin receptors: A review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab. 2014;28(1):15–23. doi:10.1016/j.beem.2013.09.003.
Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8(5):516–23. doi:10.1038/ncb1404.
Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, et al. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56(5):1387–94. doi:10.2337/db06-1580.
Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–9. doi:10.1038/nm1557.
Bjursell M, Ahnmark A, Bohlooly YM, William-Olsson L, Rhedin M, Peng XR, et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56(3):583–93. doi:10.2337/db06-1432.
Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca (2+) and AMPK/SIRT1. Nature. 2010;464(7293):1313–9. doi:10.1038/nature08991.
Ceddia RB, Somwar R, Maida A, Fang X, Bikopoulos G, Sweeney G. Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetologia. 2005;48(1):132–9. doi:10.1007/s00125-004-1609-y.
Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–95. doi:10.1038/nm788.
Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98(4):2005–10. doi:10.1073/pnas.041591798.
Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423(6941):762–9. doi:10.1038/nature01705.
Dadson K, Turdi S, Hashemi S, Zhao J, Polidovitch N, Beca S et al. Adiponectin is required for cardiac MEF2 activation during pressure overload induced hypertrophy. Journal of Molecular & Cellular Cardiology. 2014;submitted.
Civitarese AE, Ukropcova B, Carling S, Hulver M, DeFronzo RA, Mandarino L, et al. Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab. 2006;4(1):75–87. doi:10.1016/j.cmet.2006.05.002.
Gulli RA, Tishinsky JM, MacDonald T, Robinson LE, Wright DC, Dyck DJ. Exercise restores insulin, but not adiponectin, response in skeletal muscle of high-fat fed rodents. Am J Physiol Regul Integr Comp Physiol. 2012;303(10):R1062–70. doi:10.1152/ajpregu.00176.2012.
Mullen KL, Tishinsky JM, Robinson LE, Dyck DJ. Skeletal muscle inflammation is not responsible for the rapid impairment in adiponectin response with high-fat feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2010;299(2):R500–8. doi:10.1152/ajpregu.00080.2010.
Mullen KL, Smith AC, Junkin KA, Dyck DJ. Globular adiponectin resistance develops independently of impaired insulin-stimulated glucose transport in soleus muscle from high-fat-fed rats. Am J Physiol Endocrinol Metab. 2007;293(1):E83–90. doi:10.1152/ajpendo.00545.2006.
Mullen KL, Pritchard J, Ritchie I, Snook LA, Chabowski A, Bonen A, et al. Adiponectin resistance precedes the accumulation of skeletal muscle lipids and insulin resistance in high-fat-fed rats. Am J Physiol Regul Integr Comp Physiol. 2009;296(2):R243–51. doi:10.1152/ajpregu.90774.2008.
Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, Takekawa S, et al. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279(29):30817–22. doi:10.1074/jbc.M402367200.
Fang X, Palanivel R, Zhou X, Liu Y, Xu A, Wang Y, et al. Hyperglycemia- and hyperinsulinemia-induced alteration of adiponectin receptor expression and adiponectin effects in L6 myoblasts. J Mol Endocrinol. 2005;35(3):465–76. doi:10.1677/jme.1.01877.
Civitarese AE, Jenkinson CP, Richardson D, Bajaj M, Cusi K, Kashyap S, et al. Adiponectin receptors gene expression and insulin sensitivity in non-diabetic Mexican Americans with or without a family history of Type 2 diabetes. Diabetologia. 2004;47(5):816–20. doi:10.1007/s00125-004-1359-x.
Debard C, Laville M, Berbe V, Loizon E, Guillet C, Morio-Liondore B, et al. Expression of key genes of fatty acid oxidation, including adiponectin receptors, in skeletal muscle of Type 2 diabetic patients. Diabetologia. 2004;47(5):917–25. doi:10.1007/s00125-004-1394-7.
Buechler C, Wanninger J, Neumeier M. Adiponectin receptor binding proteins–recent advances in elucidating adiponectin signalling pathways. FEBS Lett. 2010;584(20):4280–6. doi:10.1016/j.febslet.2010.09.035.
Van Berendoncks AM, Garnier A, Beckers P, Hoymans VY, Possemiers N, Fortin D, et al. Exercise training reverses adiponectin resistance in skeletal muscle of patients with chronic heart failure. Heart. 2011;97(17):1403–9. doi:10.1136/hrt.2011.226373.
Wang C, Xin X, Xiang R, Ramos FJ, Liu M, Lee HJ, et al. Yin-Yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J Biol Chem. 2009;284(46):31608–15. doi:10.1074/jbc.M109.010355.
Marinho R, Ropelle ER, Cintra DE, De Souza CT, Da Silva AS, Bertoli FC, et al. Endurance exercise training increases APPL1 expression and improves insulin signaling in the hepatic tissue of diet-induced obese mice, independently of weight loss. J Cell Physiol. 2012;227(7):2917–26. doi:10.1002/jcp.23037.
Li FY, Lam KS, Xu A. Therapeutic perspectives for adiponectin: An update. Curr Med Chem. 2012;19(32):5513–23.
Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: Evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci. 2009;30(5):234–9. doi:10.1016/j.tips.2009.02.004.
Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503(7477):493–9. doi:10.1038/nature12656.
Vu V, Riddell MC, Sweeney G. Circulating adiponectin and adiponectin receptor expression in skeletal muscle: Effects of exercise. Diabetes Metab Res Rev. 2007;23(8):600–11. doi:10.1002/dmrr.778.
Farias JM, Maggi RM, Tromm CB, Silva LA, Luciano TF, Marques SO, et al. Exercise training performed simultaneously to a high-fat diet reduces the degree of insulin resistance and improves adipoR1-2/APPL1 protein levels in mice. Lipids Health Dis. 2012;11:134. doi:10.1186/1476-511X-11-134.
White PJ, Marette A. Inflammation-Induced Insulin Resistance in Obesity: When Immunity Affects Metabolic Control. In: Hawley JA, Zierath JR, editors. Physical activity and type 2 diabetes: therapeutic effects and mechanisms of action. 2008. p. 83–106.
Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. 2001;7(10):1138–43.
Zhang X, Xu A, Chung SK, Cresser JH, Sweeney G, Wong RL, et al. Selective inactivation of c-Jun NH2-terminal kinase in adipose tissue protects against diet-induced obesity and improves insulin sensitivity in both liver and skeletal muscle in mice. Diabetes. 2011;60(2):486–95. doi:10.2337/db10-0650.
Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–6.
Kaneto H, Nakatani Y, Miyatsuka T, Kawamori D, Matsuoka TA, Matsuhisa M, et al. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med. 2004;10(10):1128–32.
Shoelson SE, Lee J, Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord. 2003;27:S49–52. doi:10.1038/sj.ijo.08025010802501. Suppl 3.
Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114(6):823–7.
Stables MJ, Gilroy DW. Old and new generation lipid mediators in acute inflammation and resolution. Prog Lipid Res. 2011;50(1):35–51. doi:10.1016/j.plipres.2010.07.005.
Norling LV, Serhan CN. Profiling in resolving inflammatory exudates identifies novel anti-inflammatory and pro-resolving mediators and signals for termination. J Intern Med. 2010;268(1):15–24. doi:10.1111/j.1365-2796.2010.02235.x.
Serhan CN, Savill J. Resolution of inflammation: The beginning programs the end. Nat Immunol. 2005;6(12):1191–7. doi:10.1038/ni1276.
Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: Assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176(3):1848–59.
Chen P, Fenet B, Michaud S, Tomczyk N, Vericel E, Lagarde M, et al. Full characterization of PDX, a neuroprotectin/protectin D1 isomer, which inhibits blood platelet aggregation. FEBS Lett. 2009;583(21):3478–84. doi:10.1016/j.febslet.2009.10.004.
Balas L, Guichardant M, Durand T, Lagarde M. Confusion between protectin D1 (PD1) and its isomer protectin DX (PDX). An overview on the dihydroxy-docosatrienes described to date. Biochimie. 2013. doi:S0300-9084 (13) 00402-1 10:1016/j.biochi.2013.11.006.
Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: Fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427(6974):504. doi:10.1038/427504a 427504a.
White PJ, Arita M, Taguchi R, Kang JX, Marette A. Transgenic restoration of long-chain n-3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high-fat-fed mice. Diabetes. 2010;59(12):3066–73. doi:10.2337/db10-0054.
Gonzalez-Periz A, Planaguma A, Gronert K, Miquel R, Lopez-Parra M, Titos E, et al. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20(14):2537–9. doi:10.1096/fj.06-6250fje.
Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, et al. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARgamma-mediated mechanisms in Alzheimer’s disease models. PLoS One. 2011;6(1):e15816. doi:10.1371/journal.pone.0015816.
Claria J, Dalli J, Yacoubian S, Gao F, Serhan CN. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J Immunol. 2012;189(5):2597–605. doi:10.4049/jimmunol.1201272.
Neuhofer A, Zeyda M, Mascher D, Itariu BK, Murano I, Leitner L, et al. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes. 2013;62(6):1945–56. doi:10.2337/db12-0828.
White PJ, St-Pierre P, Charbonneau A, Mitchell PL, St-Amand E, Marcotte B, et al. Protectin DX alleviates insulin resistance by activating a myokine-liver glucoregulatory axis. Nat Med. 2014;20(6):664–9. doi:10.1038/nm.3549.
Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55(10):2688–97. doi:10.2337/db05-1404.
Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes. 2004;53(7):1643–8.
Acknowledgments
Related work in the authors laboratories is supported by operating grants from Canadian Diabetes Association (AM & GS), Heart & Stroke Foundation of Canada (GS), Canadian Institutes of Health Research (AM & GS). GS also acknowledges support via a Career Investigator Award from Heart & Stroke Fundation Ontario. AM was supported by a CIHR/Pfizer Research Chair in the pathogenesis of insulin resistance and cardiovascular diseases.
Conflict of Interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Marette, A., Liu, Y. & Sweeney, G. Skeletal muscle glucose metabolism and inflammation in the development of the metabolic syndrome. Rev Endocr Metab Disord 15, 299–305 (2014). https://doi.org/10.1007/s11154-014-9296-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-014-9296-6