Abstract
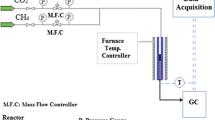
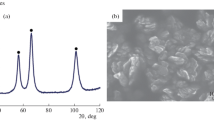
The study aims to synthesize Ni nanopowders by a facile method and understand the influence of Ni nanopowders on ammonium perchlorate (AP) thermal decomposition. Ni nanopowders with cauliflower-like shapes were prepared by simple reduction method at 80 °C with sodium hypophosphite as the reduction reagent. The morphologies, structure, composition and surface area of these nanopowders were analyzed by means of FESEM, XRD, EDS and BET. The results showed that the synthesized samples were evenly distributed and nanosized Ni with cauliflower-like shapes, with specific surface area of 9.7 m2/g. The catalytic property of Ni nanopowders on the thermal decomposition of AP was investigated by DSC tests. The results indicated that Ni nanopowders with cauliflower-like shapes had significant catalytic properties on AP thermal decomposition. Especially, Ni nanopowders with 8% addition in AP exhibited relatively higher catalytic activity, reducing decomposition temperature by about 144 °C and increasing apparent decomposition heat by 674 J/g. Kinetic analysis was carried out according to Kissinger method. The results showed that, after Ni nanopowders with cauliflower-like shapes were introduced, the activation energy of AP thermal decomposition decreased by 47% and the reaction rate constant grew by more than 115 times. In addition, the possible mechanism of AP thermal decomposition catalyzed by Ni nanopowders was briefly discussed. This study has certain theoretical and practical value for the synthesis of metal nanopowders and their catalytic application on the combustion behavior of AP-based solid propellants.







Similar content being viewed by others
Data availability
Not applicable.
References
Fertassi MA, Liu Q, Li R, Liu P, Liu J, Chen RR, Liu L, Wang J (2017) Ex situ synthesis of G/alpha-Fe2O3 nanocomposite and its catalytic effect on the thermal decomposition of ammonium perchlorate. Bull Mater Sci 40:691–698
Isert S, Xin L, Xie J, Son SF (2017) The effect of decorated graphene addition on the burning rate of ammonium perchlorate composite propellants. Combust Flame 183:322–329
Nagendra K, Raj Alexander Y, Ramakrishna PA (2017) Extinction of AP monopropellant by rapid depressurization: computational and experimental studies. Combust Flame 184:90–100
Gao K, Li GP, Luo YJ, Wang L, Shen LH, Wang G, Ren JN, Xu BB, Algadi H, Yang YY (2014) Preparation and characterization of the AP/Al/Fe2O3 ternary nano-thermites. J Therm Anal Calorim 118:43–49
Liang TX, Yang XJ, Liu B, Song RD, Xiao F, Yang YJ, Wang D, Dong MY (2023) Ammonium perchlorate@graphene oxide/Cu-MOF composites for efficiently catalyzing the thermal decomposition of ammonium perchlorate. Adv Compos Hybrid Ma 6:67
Duan HZ, Lin XY, Liu GP, Xu L, Li FS (2008) Synthesis of Ni nanoparticles and their catalytic effect on the decomposition of ammonium perchlorate. J Mater Process Technol 208:494–498
Duan GR, Yang XJ, Chen J, Huang GH, Lu LD, Wang X (2007) The catalytic effect of nanosized MgO on the decomposition of ammonium perchlorate. Powder Technol 172:27–29
Zhang YF, Ma MZ, Zhang XY, Wang BA, Liu RP (2014) Synthesis, characterization, and catalytic property of nanosized MgO flakes with different shapes. J Alloys Compd 590:373–379
Hu YH, Yang SM, Tao BW, Liu XL, Lin KF, Yang YL, Fan RQ, Xia DB, Hao DY (2019) Catalytic decomposition of ammonium perchlorate on hollow mesoporous CuO microspheres. Vacuum 159:105–111
Alizadeh-Gheshlaghi E, Shaabani B, Khodayari A, Azizian-Kalandaragh Y, Rahimi R (2012) Investigation of the catalytic activity of nano-sized CuO, Co3O4 and CuCo2O4 powders on thermal decomposition of ammonium perchlorate. Powder Technol 217:330–339
Ayoman E, Hosseini SG (2016) Synthesis of CuO nanopowders by high-energy ball-milling method and investigation of their catalytic activity on thermal decomposition of ammonium perchlorate particles. J Therm Anal Calorim 123:1213–1224
Yu CP, Zhang WC, Gao Y, Chen YJ, Ma KF, Ye JH, Shen RQ, Yang Y (2018) Shape-controlled syntheses of Co3O4 nanowires arrays with excellent catalytic performances upon ammonium perchlorate decomposition. Mater Res Bull 97:483–489
Sharmaa JK, Srivastavaa P, Singha G, Shaheer Akhtar M, Ameen S (2015) Green synthesis of Co3O4 nanoparticles and their applications in thermal decomposition of ammonium perchlorate and dye-sensitized solar cells. Mater Sci Eng B 193:181–188
Hosseini SG, Toloti SJH, Babaei K, Ghavi A (2016) The effect of average particle size of nano-Co3O4 on the catalytic thermal decomposition of ammonium perchlorate particles. J Therm Anal Calorim 124:1243–1254
Zhang M, Zhao FQ, Yang YJ, An T, Qu WG, Li H, Zhang JK, Li N (2020) Catalytic activity of ferrates (NiFe2O4, ZnFe2O4 and CoFe2O4) on the thermal decomposition of ammonium perchlorate. Propellants Explos Pyrotech 45:463–471
Chen J, He SM, Liu YS, Qiao ZQ, Huang B, Li XD, Hao QL, Huang H, Yang GC (2021) Highly active catalysts based on 3D hierarchically ordered porous carbon with entrapped Fe2O3 nanoparticles for the thermal decomposition of ammonium perchlorate. Appl Surf Sci 538:148148
Singh S, Singh G, Kulkarni N, Mathe VL, Bhoraskar SV (2015) Synthesis, characterization and catalytic activity of Al/Fe2O3 nanothermite. J Therm Anal Calorim 119:309–317
Xu PF, Lu YW, Ye P, Wang Q, Guo CP (2021) In situ synthesis of copper stearate/ammonium perchlorate shell–core composite for self-catalytic thermal decomposition. J Therm Anal Calorim 146:11–15
Zheng SJ, Liu J, Wang YK, Li FS, Xiao L, Ke X, Hao GZ, Jiang W, Li D, Li Y, Lan ZG (2018) Effect of aluminum morphology on thermal decomposition of ammonium perchlorate. J Therm Anal Calorim 134:1823–1828
Chen WF, Li FS, Liu LL, Li YQ (2006) Synthesis of Nano-sized yttria via a sol-gel process based on hydrated yttrium nitrate and ethylene glycol and its catalytic performance for thermal decomposition of NH4 C1O4. J Rare Earth 24:543–548
Li R, Li XD, Yang GC, Guo CP, Zhang LQ, Hao CC, Zhu WK (2023) Defective-activated-carbon-encapsulated Co as a super reactive catalyst for combustion of ammonium perchlorate. Appl Surf Sci 615:156349
Inokawa H, Ichikawa T, Miyaoka H (2015) Catalysis of nickel nanoparticles with high thermal stability for ammonia decomposition. Appl Catal A: Gen 491:184–188
Kamal T, Asiri Abdullah M, Nauman A (2021) Catalytic reduction of 4-nitrophenol and methylene blue pollutants in water by copper and nickel nanoparticles decorated polymer sponges. Spectrochim Acta Part A 261:120019
Kiran S, Rafique MA, Iqbal S, Nosheen S, Naz S, Rasheed A (2020) Synthesis of nickel nanoparticles using citrullus colocynthis stem extract for remediation of reactive yellow 160 dye. Environ Sci Pollut R 27:32998–33007
Lanje AS, Sharma SJ, Pode RB (2010) Magnetic and electrical properties of nickel nanoparticles prepared by hydrazine reduction method. Arch Phys Res 1(1):49–56
Chen YJ, Li XY, Liu X, Liu C, Zhang ZH, Sang WX, Chen R (2019) Study on removal of U(VI) in aqueous solution with nano-zero-valent nickel. J Nonferrous Met 2:71–75
El komy GM, Abomostafa H, Azab AA, Selim MM (2019) Innovative synthesis of nickel nanoparticles in polystyrene matrix with enhanced optical and magnetic properties. J Inorg Organomet 29:1983–1994
Chou KS, Huang KC (2001) Studies on the chemical synthesis of nanosized nickel powder and its stability. J Nanopart Res 3:127–132
Liu SN, Tam SK, Ng KM (2021) Dual-reductant synthesis of nickel nanoparticles for use in screen-printing conductive paste. J Nanopart Res 23:78
Eluri R, Paul B (2012) Synthesis of nickel nanoparticles by hydrazine reduction: mechanistic study and continuous flow synthesis. J Nanopart Res 14:800
Yakymovych A, Ipser H (2017) Synthesis and characterization of pure Ni and Ni-Sn intermetallic nanoparticles. Nanoscale Res Lett 12:142
Kytsya AR, Bazylyak LI, Zavaliy IYu, Verbovytskyy YuV, Zavalij P (2022) Synthesis, structure and hydrogenation properties of Ni-Cu bimetallic nanoparticles. Appl Nanosci 12:1183–1190
Rana MZ, Mehmood M, Ahmad J, Aslam M, Hasanain SK, Hameed S (2011) Synthesis of nickel nanoparticles in silica by alcogel electrolysis. J Nanopart Res 13:375–384
Bouremana A, Mouaci S, Berriah A, Boutebina Z, Manseri A, Bensouilah A (2022) High yield solvothermal synthesis of Ni nanoparticles: structural, microstructural, and magnetic properties. J Nanopart Res 24:204
Liu DY, Ren S, Wang GS, Wen LS, Yu J (2009) Rapid synthesis and morphology control of nickel powders via a microwave-assisted chemical reduction method. J Mater Sci 44:108–113
Xu W, Liew KY, Liu H, Huang T, Sun C, Zhao Y (2008) Microwave-assisted synthesis of nickel nanoparticles. Mater Lett 62:2571–2573
Zhang T, Shi HB, Zhang YB, Liu Q, Fei WY, Wang T (2021) Hollow flower-like nickel particles as the promoter of ammonium perchlorate-based solid propellant. Appl Surf Sci 552:149506
Nourine M, Boulkadid MK, Touidjine S, Akbi H, Belkhiri S (2023) Preparation of surface modified ammonium perchlorate with green iron oxide nitrocellulose nanocomposite for catalytic thermal decomposition of ammonium perchlorate. Mater Chem Phys 303:127784
Lu YW, Chen J, Wang RH, Xu PF, Zhang XQ, Gao B, Guo CP, Yang GC (2019) Bio-inspired Cu-alginate to smartly enhance safety performance and the thermal decomposition of ammonium perchlorate. Appl Surf Sci 470:269–275
Luo HK, Yin ZW, Zhao YX, Li XJ, Sun TQ, Yang SJ (2023) Interfacial effects of Fe2O3@Co3O4 on the thermal decomposition of ammonium perchlorate. Mater Res Bull 165:112291
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29:1702–1706
Dhyani V, Kumar J, Bhaskar T (2017) Thermal decomposition kinetics of sorghum straw via thermogravimetric analysis. Bioresour Technol 245:1122–1129
Ceylan S, Kazan D (2015) Pyrolysis kinetics and thermal characteristics of microalgae nannochloropsis oculata and tetraselmis sp. Bioresour Technol 187:1–5
Hosseini SG, Sheikhpour A, Keshavarz MH, Tavangar S (2016) The effect of metal oxide particle size on the thermal behavior and ignition kinetic of Mg–CuO thermite mixture. Thermochim Acta 626:1–8
Wen T, Feng Y, Bi YQ, Xu L, Guo CP (2022) 3D porous nano Co3O4/C composite catalyst for the thermal decomposition of ammonium perchlorate. Mater Today Commun 33:104294
Zhang YF, Song AJ, Ma DQ, Ma MZ (2021) The catalytic decomposition and kinetic analysis of ammonium perchlorate on MgO nanoflakes. J Phys Chem Solids 157:110205
Bekhouche S, Trache D, Abdelaziz A, Tarchoun AF, Boukeciat H (2023) Effect of fluorine-containing thermite coated with potassium perchlorate on the thermal decomposition behavior and kinetics of ammonium perchlorate. Thermochim Acta 720:179413
Dourari M, Tarchoun AF, Trache D, Abdelaziz A, Barkat T, Tiliouine R, Bekhouche S, Bessa W (2023) Elucidating the effect of nitrocellulose-encapsulated MgAl–CuO on the thermal behavior of double base propellant based on nitrocellulose and diethylene glycol dinitrate. Reac Kinet Mech Cat. https://doi.org/10.1007/s11144-023-02448-2
Xuan CL, Zhao FQ, Xiao L, Hao GZ, Jiang W (2018) Preparation of nano-sized CuFe2O4 and its catalytic effects on the thermal decomposition of ammonium perchlorate. J Solid Rocket Technol 41:343–348
Chen T, Hu Y, Zhang C, Gao Z (2021) Recent progress on transition metal oxides and carbon-supported transition metal oxides as catalysts for thermal decomposition of ammonium perchlorate. Def Technol 17:1471–1485
Shi Z, Tang C, Xu Y, Hu Y, Zhang J, Zhu Z, Zheng J, Fan R, Xia D, Yang Y (2022) New insights into the catalytic decomposition of ammonium perchlorate and decomposition mechanism by Nano-CuO dispersed in graphite-carbon nitride nanosheet composites. Chem Nano Mat 8:e202200118
Mani G, Jos J, Radhakrishnan Nair P, Mathew S (2021) Investigation of kinetic parameters for ammonium perchlorate thermal decomposition in presence of gCN/CuO by TG-MS analysis and kinetic compensation correction. J Solid State Chem 301:122301
Bircomshaw LL, Newman BH (1955) Thermal decomposition of ammonium perchlorate. Proc R Soc A 227:228–237
Galwey AK, Jacobs P (1960) Thermal decomposition of ammonium perchlorate at low-temperatures. Proc R Soc A 254:455–469
Acknowledgements
This work was supported by the NSFC (Grant No. 52071278/51827801), the National Key Research and Development Program of China (Grant No. 2018YFA0703603), PhD Fund Project (Grant No. 19YB1001) and Science and Technology Research Project of Colleges and Universities in Hebei Province (Grant No. ZC2023173).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Li, Z. & Ma, M. Synthesis of Ni nanopowders with cauliflower-like shapes and their catalytic property on the thermal decomposition of ammonium perchlorate. Reac Kinet Mech Cat 136, 2327–2341 (2023). https://doi.org/10.1007/s11144-023-02473-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02473-1