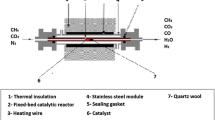
Abstract
Dynamics of the non-oxidative conversion of methane in a moving mixture of the catalyst nanoparticles and the reacting gas is under study using the methods of mathematical modeling. Considered compact mechanism of the reaction takes into account activation of methane both on the surface of the catalyst nanoparticles (NPs) and in the gas phase, with the production of C2Hn, n = 2, 4, 6, C6H6 and H2. At this, the desorption of H or CH3 increases the rates of the reactions in the gas phase. Under the parametric analysis of the kinetic model, we defined some values of the temperature, the energy of catalytic activation of methane and the concentration of the catalyst NPs at which both the methane conversion and the C2H6 and C2H4 selectivity increase. Within the non-isothermal model of the flow fluidized-like bed wall-less reactor we studied the influence of the mass and heat transfer of the conversion of methane and mole fractions of the products of the reaction. In results of the studies, we describe the case when due to the activation of methane the catalyst NPs are cooling, which lead to cooling of the gas via the convective heat transfer. We also simulate the case of the two-temperature regime (the catalyst NPs are colder than the gas phase), which allows maintaining a limited set of products of the reaction, namely, hydrogen, ethane and ethylene.





Similar content being viewed by others
References
Chen C-J, Back MH, Back RA (1975) The thermal decomposition of methane. I. Kinetics of the primary decomposition to C2H6 + H2; rate constant for the hondmogeneous unimolecular dissociation of methane and its pressure dependence. Can J Chem 53:3580–3590
Dean AM (1990) Detailed kinetic modeling of autocatalysis in methane pyrolysis. J Phys Chem 94:1432–1439
Albright LF, Crynes BL, Corcoran WH (1983) Pyrolysis. Theory and industrial practice. Academic Press, New York
Younessi-Sinaki M, Matida EA, Hamdullahpur F (2009) Kinetic model of homogeneous thermal decomposition of methane and ethane. Int J Hydrogen Energy 34:3710–3716
Kumar G, Lau SLJ, Krcha DM, Janik MJ (2016) Correlation of methane activation and oxide catalyst reducibility and its implications for oxidative coupling. ACS Catal 6(3):1812–1821
Wang B, Albarracín-Suazo S, Pagán-Torres Y, Nikolla E (2017) Advances in methane conversion processes. Catal Today 285:147–158
Quiceno R, Pérez-Ramírez J, Warnatz J, Deutschmann O (2006) Modeling the high-temperature catalytic partial oxidation of methane over platinum gauze: detailed gas-phase and surface chemistries coupled with 3D flow field simulations. Appl Cat A 303:166–176
Burghgraef H, Jansen APJ, van Santen RA (1995) Methane activation and dehydrogenation on nickel and cobalt: a computational study. Surf Sci 324:345–356
Rostrup-Nielsen J, Trimm DL (1977) Mechanisms of carbon formation on nickel-containing catalysts. J Catal 48:155–165
Torres D, de Llobet S, Pinilla JL, Lázaro ML, Suelves I, Moliner R (2012) Hydrogen production by catalytic decomposition of methane using a Fe-based catalyst in a fluidized bed reactor. J Nat Gas Chem 21:367–373
Guo X, Fang G, Li G, Ma H, Fan H, Yu L, Ma C, Wu X, Deng D, Wei M, Tan D, Si R, Zhang S, Li J, Sun L, Tang Z, Pan X, Bao X (2014) Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 344(6184):616–619
Eggart D, Huang X, Zimina A, Yang J, Pan Y, Pan X, Grunwaldt J-D (2022) Operando XAS study of Pt-doped CeO2 for the nonoxidative conversion of methane. ACS Catal 12(7):3897–3908
Dipu AL, Ohbuchi Sh, Nishikawa Y, Iguchi Sh, Ogihara H, Yamanaka I (2020) Direct nonoxidative conversion of methane to higher hydrocarbons over silica-supported nickel phosphide catalyst. ACS Catal 10(1):375–379
Han SJ, Gebreyohannes TG, Woolee S, Kim SK, Kim HW, Shin J, Kim YT (2023) Methane direct conversion to olefins, aromatics, and hydrogen over silica entrapped bimetallic MeFe-SiO2 (Me = Co, Ni, Pd, Pt) catalysts. Molec Catal 535:112864
Donazzi A, Livio D, Maestri M, Beretta A, Groppi G, Tronconi E, Forzatti P (2011) Synergy of homogeneous and heterogeneous chemistry probed by in situ spatially resolved measurements of temperature and composition. Angew Chem 50(17):3943–3946
Li L, Borry RW, Iglesia E (2001) Reaction-transport simulations of non-oxidative methane conversion with continuous hydrogen removal—homogeneous–heterogeneous reaction pathways. Chem Eng Sci 56:1869–1881
Zhuzhgov AV, Zaitseva NA, Kostyukov AI, Baronskiy MG, Nashivochnikov AA, Snytnikov VN (2023) Effect of the laser-synthesized γ-Al2O3 support on the activity of Fe/γ-Al2O3 nanopowders in dehydrogenation of isobutane. Mol Catal 535:112892
Kostyukov AI, Zaitseva NA, Baronskiy MG, Nashivochnikov AA, Snytnikov VN (2022) Catalytic activity of laser-synthesized CrOx/Al2O3 nanocatalysts with different particle sizes in isobutane dehydrogenation. J Nanoparticle Res 24:144
Stadnichenko OA, Nurislamova LF, Masyuk NS, Snytnikov VN, Snytnikov VN (2018) Radical mechanism for the gas-phase thermal decomposition of propane. Reac Kinet Mech Catal 123:607–624
Masyuk N, Sherin A, Snytnikov VN (2018) Effect of infrared laser radiation on gas-phase pyrolysis of ethane. J Anal Appl Pyrol 134:122–129
Postma RS, Mendes PSF, Pirro L, Banerjee A, Thybaut JW, Lefferts L (2023) Modelling of the catalytic initiation of methane coupling under non-oxidative conditions. Chem Eng J 454:140273
Kim SK, Kim HW, Han SJ, Lee SW, Shin J, Kim YT (2020) Mechanistic and microkinetic study of non-oxidative methane coupling on a single-atom iron catalyst. Commun Chem 3:58
Toraman HE, Alexopoulos K, Oh SC, Cheng S, Liu D, Vlachos DG (2021) Ethylene production by direct conversion of methane over isolated single active centers. Chem Eng J 420:130493
Schultz RH, Elkind JL, Armentrout PB (1988) Electronic effects in C-H and C-C bond activation. State-specific reactions of FE+ (6D,4F) with methane, ethane, and propane. J Am Chem Soc 110:411–423
Hao J, Schwach P, Fang G, Guo X, Zhang H, Shen H, Huang X, Eggart D, Pan X, Bao X (2019) Enhanced Methane conversion to olefins and aromatics by H-donor molecules under nonoxidative condition. ACS Catal 9:9045–9050
Hao J, Schwach P, Li L, Guo X, Weng J, Zhang H, Shen H, Fang G, Huang X, Pan X, Xiao C, Yang X, Bao X (2021) Direct experimental detection of hydrogen radicals in non-oxidative methane catalytic reaction. J Energy Chem 52:372–376
NIST Chemical Kinetics Database. https://kinetics.nist.gov/kinetics/index.jsp.
Han SJ, Lee SW, Kim HW, Kim SK, Kim YT (2019) Nonoxidative direct conversion of methane on silica-based iron catalysts: effect of catalytic surface. ACS Catal 9:7984–7997
Peskova EE (2021) Numerical modeling of subsonic axisymmetric reacting gas flows. J Phys Conf Ser 2057:012071
Acknowledgements
This research was funded by Russian Science Foundation, grant number 21-19-00429.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lashina, E.A., Peskova, E.E. & Snytnikov, V.N. Mathematical modeling of the homogeneous-heterogeneous non-oxidative CH4 conversion: the role of gas-phase H or CH3. Reac Kinet Mech Cat 136, 1775–1789 (2023). https://doi.org/10.1007/s11144-023-02442-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02442-8