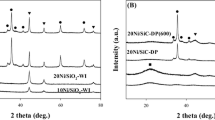
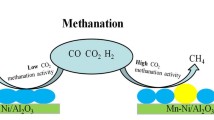
Abstract
The Mn promoted Ni catalysts were developed and applied in CO methanation reaction. The 10%Ni/SiO2 catalyst exhibits poor initial CO conversion (32.8%) and rapid deactivation with the highest methane selectivity during CO methanation reaction. In contrast, the Mn 4%Mn-10%Ni/SiO2 catalyst shows dramatically increased initial CO conversion, which is up to 94.5% with 90.0% methane selectivity. Besides, the apparent activation energy, Ea value, of 4%Mn-10%Ni/SiO2 was calculated to be 73.1 kJ/mol according to Arrhenius equation, which is much lower than that of 10%Ni/SiO2 catalyst as 139.1 kJ/mol. Based on various characterization results, including in situ XPS and in situ CO-DRIFTS, it is found that the added Mn significantly improves the dispersion of the supported nickel, suppresses the sintering of nickel particles and forms more adsorbed CO species of three-fold carbonyl species, resulting in higher CO conversion and good stability during CO methanation reaction.







Similar content being viewed by others
References
Hu DC, Gao JJ, Ping Y, Jia LH, Gunawan P, Zhong ZY, Xu GW, Gu FN, Su FB (2012) Enhanced Investigation of CO Methanation over Ni/Al2O3 catalysts for synthetic natural gas production. Ind Eng Chem Res 51:4875–4886
Wang H, Fang YZ, Liu Y, Bai X (2012) Perovskite LaFeO3 supported bi-metal catalyst for syngas methanation. J Nat Gas Chem 6:745–752
Barrientos J, Lualdi M, Boutonnet M (2014) Deactivation of supported nickel catalysts during CO methanation. Appl Catal A 486:143–149
Italiano C, Llorca J, Pino L, Ferraro M, Antonucci V, Vita A (2020) CO and CO2 methanation over Ni catalysts supported on CeO2, Al2O3 and Y2O3 oxides. Appl Catal B: Environ 264:118494
Lin XH, Yang K, Si RR, Chen X, Dai WX, Fu XZ (2014) Photo-assisted catalytic methanation of CO in H2-rich stream over Ru/TiO2. Appl Catal B 147:585–591
Liu JX, Su HY, Li WX (2013) Structure sensitivity of CO methanation on Co (0001), (1012) and (1120) surfaces: density functional theory calculations. Catal Today 215:36–42
Weatherbee GD, Bartholomew CH (1982) Hydrogenation of CO2 on group VIII metals: II. Kinetics and mechanism of CO2 hydrogenation on nickel. J Catal 77:460–472
Guo X, Traitangwong A, Hu M, Zuo C, Meeyoo V, Peng Z, Li C (2018) Carbon dioxide methanation over nickel-based catalysts supported on various mesoporous material. Energy Fuels 32:3681–3689
Aziz MAA, Jalil AA, Triwahyono S, Mukti RR, Taufiq-Yap YH, Sazegar MR (2014) Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl Catal B: Environ 147:359–368
Aziz MAA, Jalil AA, Triwahyono S, Ahmad A (2015) CO2 methanation over heterogeneous catalysts: recent progress and future prospects. Green Chem 17:2647–2663
Chen Y, Qiu B, Liu Y, Zhang Y (2020) An active and stable nickel-based catalyst with embedment structure for CO2 methanation. Appl Catal B: Environ 269:118801
Hu CW, Yao J, Yang HQ, Chen Y, Tian AM (1997) On the inhomogeneity of low nickel loading methanation catalyst. J Catal 166:1–7
Hwang S, Lee J, Hong UG, Seo JG, Jung JC, Koh DJ, Lim H, Byun C, Song IK (2011) Methane production from carbon monoxide and hydrogen over nickel–alumina xerogel catalyst: effect of nickel content. J Ind Eng Chem 17:154–157
GaoJ JC, Li J, Zhang M, Gu F, Xu G, Zhong Z, Su F (2013) Effect of nickel nanoparticle size in Ni/α-Al2O3 on CO methanation reaction for the production of synthetic natural gas. Catal Sci Technol 3:2009–2015
Munnik P, Velthoen ME, de Jongh PE, de Jong KP, Gommes CJ (2014) Nanoparticle growth in supported nickel catalysts during methanation reaction—larger is better. Angew Chem Int Ed 126:9647–9651
Deleitenburg C, Trovarelli A (1995) Metal-support interactions in Rh/CeO2, Rh/TiO2, and Rh/Nb2O5 catalysts as inferred from CO2 methanation activity. J Catal 156:171–174
Vannice MA, Wang SY, Moon SH (1981) The effect of SMSI (strong metal-support interaction) behavior on CO adsorption and hydrogenation on Pd catalysts: I. IR spectra of adsorbed CO prior to and during reaction conditions. J Catal 71:152–166
AzizMAA JAA, Triwahyono S, Sidik SM (2014) Methanation of carbon dioxide on metal-promoted mesostructured silica nanoparticles. Appl Catal A 486:115–122
Qin Z, Ren J, Miao M, Li Z, Lin J, Xie KC (2015) The catalytic methanation of coke oven gas over Ni-Ce/Al2O3 catalysts prepared by microwave heating: Effect of amorphous NiO formation. Appl Catal B: Environ 164:18–30
Li XC, Hu QH, Yang YF, Wang Y, He F (2012) Studies on stability and coking resistance of Ni/BaTiO3–Al2O3 catalysts for lower temperature dry reforming of methane (LTDRM). Appl Catal A: Gen 413:163–169
Lv X, Chen JF, Tan Y, Zhang Y (2012) A highly dispersed nickel supported catalyst for dry reforming of methane. Catal Commun 20:6–11
Agnelli M, Kolb M, Mirodatos C (1994) Co Hydrogenation on a nickel catalyst: 1. Kinetics and modeling of a low-temperature sintering process. J Catal 148:9–21
Shen WM, Dumesic JA, Hill CG (1981) Criteria for stable Ni particle size under methanation reaction conditions: nickel transport and particle size growth via nickel carbonyl. J Catal 68:152–165
Bai YX, Zhang JF, Yang GH, Zhang QD, Pan JX, Xie HJ, Liu XC, Han YZ, Tan YS (2018) Insight into the nanoparticle growth in supported Ni catalysts during the early stage of CO hydrogenation reaction: the important role of adsorbed CO molecules. ACS Catal 8:6367–6374
Younas M, Kong LL, Bashir MJK, Nadeem H, Shehzad A, Sethupathi S (2016) Recent advancements, fundamental challenges, and opportunities in catalytic methanation of CO2. Energy Fuels 30:8815–8831
Beierlein D, Häussermann D, Pfeifer M, Schwarz T, Stöwe K, Traa Y, Klemm E (2019) Is the CO2 methanation on highly loaded Ni-Al2O3 catalysts really structure-sensitive. Appl Catal B: Environ 247:200–219
Wu HC, Chang YC, Wu JH, Lin JH, Lin IK, Chen CS (2015) Methanation of CO2 and reverse water gas shift reactions on Ni/SiO2 catalysts: the influence of particle size on selectivity and reaction pathway. Catal Sci Technol 5:4154–4163
Liu J, Li CM, Wang F, He S, Chen H, Zhao YF, Wei M, Evans DG, Duan X (2013) Enhanced low-temperature activity of CO2 methanation over highly-dispersed Ni/TiO2 catalyst. Catal Sci Technol 3:2627–2633
Zhou WG, Liu JY, WuX CJF, Zhang Y (2015) An effective Co/MnOx catalyst for forming light olefins via Fischer-Tropsch synthesis. Catal Commun 60:76–81
Liu Y, Chen JF, Bao J, Zhang Y (2015) Manganese-modified Fe3O4 microsphere catalyst with effective active phase of forming light olefins from syngas. ACS Catal 5:3905–3909
Morales F, de Groot FMF, Gijzeman OLJ, Mens AD, Stephan O, Weckhuysen BM (2005) Mn promotion effects in Co/TiO2 Fischer-Tropsch catalysts as investigated by XPS and STEM-EELS. J Catal 230:301–308
Feltes TE, Espinosa-Alonso L, de Smit E, D’Souza L, Meyer RJ, Weckhuysen BM, Regalbuto JR (2010) Selective adsorption of manganese onto cobalt for optimized Mn/Co/TiO2 Fischer-Tropsch catalysts. J Catal 270:95–102
Choudhury MBI, Ahmed S, Shalabi MA, Inui T (2006) Preferential methanation of CO in a syngas involving CO2 at lower temperature range. Appl Catal A: Gen 314:47–53
Jia X, Zhang X, Rui N, Hu X, Liu CJ (2019) Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl Catal B: Environ 244:159–169
Wang Y, Yao L, Wang YN, Wang SH, Zhao Q, Mao DH, Hu CW (2018) Low-temperature catalytic CO2 dry reforming of methane on Ni-Si/ZrO2 catalyst. ACS Catal 8:6495–6506
Czekaj I, Loviat F, Raimondi F, Wambach J, Biollaz S, Wokaun A (2007) Characterization of surface processes at the Ni-based catalyst during the methanation of biomass-derived synthesis gas: X-ray photoelectron spectroscopy (XPS). Appl Catal A: Gen 329:68–78
Liu Y, Sheng W, Hou ZG, Zhang Y (2018) Homogeneous and highly dispersed Ni–Ru on a silica support as an effective CO methanation catalyst. RSC Adv 8:2123–2131
Yan Y, Dai Y, Yang Y, Lapkin AA (2018) Improved stability of Y2O3 supported Ni catalysts for CO2 methanation by precursor-determined metal-support interaction. Appl Catal B: Environ 237:504–512
Vogt C, Groeneveld E, Kamsma G, Nachtegaal M, Lu L, Kiely C, Berben P, Meirer F, Weckhuysen B (2018) Unravelling structure sensitivity in CO2 hydrogenation over nickel. Nat Catal 1:127–134
Acknowledgements
This work is supported by National Natural Science Foundation of P. R. China (U20B2022, 52270096 and 22078006), Beijing Nova Program (Z201100006820022) and Bingtuan Science and Technology Program (2021DB006). The financial supports from National Energy Investment Group Corporation Limited (CF9300220001) and National Key Research and Development Program of China (2018YFE0106700) are appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hou, Z., Chen, Y., Wang, C. et al. The promotional effects of Mn on Ni/SiO2 catalysts for CO methanation. Reac Kinet Mech Cat 136, 587–601 (2023). https://doi.org/10.1007/s11144-023-02377-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02377-0