Abstract
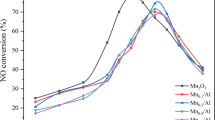
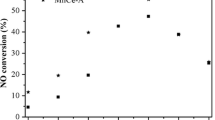
In this paper, a single-phase supported MnO2/Al2O3 catalyst and Ce-doped MnCe/Al catalyst system were used for the NO-SCO experiment with MnO2 as the main active component. Furthermore, the effect of loading and Ce doping on active oxygen species and the important factors related to catalyst activity was revealed. The MnO2 catalyst obtained the best activity at 300 °C, corresponding to a NO conversion rate of 79.4%. After MnO2 was loaded on the γ-Al2O3, the activity of the Mn/Al catalysts was not as good as that of the MnO2 catalyst, and the temperature window of Mn/Al shifted to the high-temperature region with the increase of the loading ratio. The relative content of adsorbed oxygen Oα of Mn/Al catalysts was lower than that of MnO2, resulting in poor activity. Ce-doped Mn0.23Ce0.023/Al catalysts exhibited good low-temperature activity. The introduction of the Ce element enhanced the dispersion of Mn species on the catalyst surface, the relative content of surface adsorbed oxygen Oα increased, and the low-temperature activity and mobility of surface oxygen species were enhanced. The Mn0.23Ce0.023/Al catalyst was endowed with both a high specific surface area and good pore structure, as well as a large oxygen storage capacity and excellent surface oxygen species.


Similar content being viewed by others
Data availability
All data, models, and code generated or used during the study appear in the submitted article.
References
Usmani M, Kondal A, Wang J, Jutla A (2020) Environmental association of burning agricultural biomass in the Indus River basin. GeoHealth. https://doi.org/10.1029/2020GH000281
Zara M, Boersma KF, Eskes HJ, Gon HA, Arellano JV, Krol MC, Swaluw EV, Schuch W, Velders GJ (2021) Reductions in nitrogen oxides over the Netherlands between 2005 and 2018 observed from space and on the ground: decreasing emissions and increasing O6 indicate changing NOx chemistry. Atmos Environ X 9:100104
Zhou Z, Liu X, Hu Y, Xu J, Cao XE, Liao Z, Xu M (2018) Investigation on synergistic oxidation behavior of NO and Hg0 during the newly designed fast SCR process. Fuel 225:134–139. https://doi.org/10.1016/J.FUEL.2018.03.152
Zhao H, Hill AJ, Ma L, Bhat A, Jing G, Schwank JW (2021) Progress and future challenges in passive NO adsorption over Pd/zeolite catalysts. Catal Sci Technol 2021(11):5986–6000
Zhang L, Wang X, Lai W, Cheng X, Zhao K (2014) Removal dynamics of nitric oxide (NO) pollutant gas by pulse-discharged plasma technique. Sci World J 2014:653576. https://doi.org/10.1155/2014/653576
Bao J, Li K, Ning P, Wang C, Song X, Luo Y, Sun X (2021) Study on the role of copper converter slag in simultaneously removing SO2 and NOx using KMnO4/copper converter slag slurry. J Environ Sci 108:33–43
Zheng C, Xu C, Zhang Y, Zhang J, Gao X, Luo Z, Cen K (2014) Nitrogen oxide absorption and nitrite/nitrate formation in limestone slurry for WFGD system. Appl Energy 129:187–194
Shao J, Ma Q, Wang Z, Tang H, He Y, Zhu Y, Cen K (2019) A superior liquid phase catalyst for enhanced absorption of NO2 together with SO2 after low temperature ozone oxidation for flue gas treatment. Fuel 247:1–9
Yun TG, Heo Y, Bin Bae H, Chung S (2021) Elucidating intrinsic contribution of d-orbital states to oxygen evolution electrocatalysis in oxides. Nat Commun 12(1):824
Liu Y, Gao F, Yi H, Yang C, Zhang R, Zhou Y, Tang X (2020) Recent advances in selective catalytic oxidation of nitric oxide (NO-SCO) in emissions with excess oxygen: a review on catalysts and mechanisms. Environ Sci Pollut Res 28:2549–2571
Boningari T, Pappas DK, Smirniotis PG (2018) Metal oxide-confined interweaved titania nanotubes M/TNT (M= Mn, Cu, Ce, Fe, V, Cr, and Co) for the selective catalytic reduction of NOx in the presence of excess oxygen. J Catal 365:320–333
Wang H, Chen H, Wang Y, Lyu YK (2019) Performance and mechanism comparison of manganese oxides at different valence states for catalytic oxidation of NO. Chem Eng J 361:1161–1172
Shao J, Lin F, Huang Y, Wang Z, Li Y, Chen G, Cen K (2020) MnO fabrication with rational design of morphology for enhanced activity in NO oxidation and SO2 resistance. Appl Surf Sci 503:144064
Wu Z, Tang N, Xiao L, Liu Y, Wang H (2010) MnO(x)/TiO(2) composite nanoxides synthesized by deposition-precipitation method as a superior catalyst for NO oxidation. J Colloid Interface Sci 352(1):143–148
Rahman SM, Tahmasebi A, Moghtaderi B, Yu J (2020) Kinetics and mechanism of catalytic oxidation of no in coal combustion flue gas over co-doped Mn–Ti oxide catalyst. Energy Fuels 34:6052–6058
Lei Z, Hao S, Zhang L, Yang J, Yusu W (2020) MnOx-CuOx cordierite catalyst for selective catalytic oxidation of the NO at low temperature. Environ Sci Pollut Res 27:23695–23706
Guo R, Chen Q, Ding H, Wang Q, Pan W, Yang N, Lu C (2015) Preparation and characterization of CeOx@MnOx core–shell structure catalyst for catalytic oxidation of NO. Catal Commun 69:165–169
Coelho AC, Rocha G, Santos PD, Santos HD, Kiyohara PK (2008) Specific surface area and structures of aluminas from fibrillar pseudoboehmite. Materia-rio De Janeiro 13:329–341
Wang D, Li H, Dong N, Hui S (2021) Effect of calcination conditions on MnOx/Al2O3 Catalytic Efficiency for NO Oxidation. J Environ Eng 147:04021039
Lykaki M, Pachatouridou E, Carabineiro SA, Iliopoulou E, Andriopoulou C, Kallithrakas-Kontos N, Konsolakis M (2018) Ceria nanoparticles shape effects on the structural defects and surface chemistry: implications in CO oxidation by Cu/CeO2 catalysts. Appl Catal B 230:18–28
Mou C, Li H, Dong N, Hui S, Wang D (2021) Effect of ce addition on adsorption and oxidation of NO over MnO x /Al2O3. Adsorp Sci Technol. https://doi.org/10.1155/2021/3131309
Chen H, Wang Y, Lv Y (2016) Catalytic oxidation of NO over MnO2 with different crystal structures. RSC Adv 6:54032–54040
Chen G, Hong D, Xia H, Sun W, Shao S, Gong B, Wang S, Wu J, Wang X, Dai Q (2022) Amorphous and homogeneously Zr-doped MnOx with enhanced acid and redox properties for catalytic oxidation of 1,2-Dichloroethane. Chem Eng J. https://doi.org/10.1016/j.cej.2021.131067
Salmas CE, Stathopoulos VN, Pomonis PJ, Androutsopoulos GP (2002) Pore structure−chemical composition interactions of new high surface area manganese based mesoporous materials. materials preparation, characterization, and catalytic activity. Langmuir 18:423–432
Li Z, Meng M, Li Q, Xie Y, Hu T, Zhang J (2010) Fe-substituted nanometric La0.9K0.1Co1−xFexO3−δ perovskite catalysts used for soot combustion, NOx storage and simultaneous catalytic removal of soot and NOx. Chem Eng J 164:98–105
Tang X, Wang C, Gao F, Han W, Yi H, Zhao S, Zhou Y, Liu Y (2020) Mn-Fe-Ce multiple oxides with Al2O3 coating supported onto honeycomb cordierite monoliths for NO catalytic oxidation. Colloids Surf, A 611:125790
Zhang X, Zhao H, Song Z, Liu W, Zhao J, Zhao M, Xing Y (2019) Insight into the effect of oxygen species and Mn chemical valence over MnOx on the catalytic oxidation of toluene. Appl Surf Sci 493:9–17
Tran QN, Martinović F, Ceretti M, Esposito S, Bonelli B, Paulus W, di Renzo F, Deorsola FA, Bensaid S, Pirone R (2020) Co-doped LaAlO3 perovskite oxide for NOx-assisted soot oxidation. Appl Catal A 589:117304
Wang Z, Lin F, Jiang S, Qiu K, Kuang M, Whiddon R, Cen K (2016) Ceria substrate–oxide composites as catalyst for highly efficient catalytic oxidation of NO by O2. Fuel 166:352–360
Li J, Shi L, Feng G, Shi Z, Sun C, Kong D (2020) Selective hydrogenation of naphthalene over γ-Al2O3-supported NiCu and NiZn bimetal catalysts. Catalysts 10(10):1215
Xu GY, Guo XL, Cheng X, Yu J, Fang B (2021) A review of Mn-based catalysts for low-temperature NH3-SCR: NOx removal and H2O/SO2 resistance. Nanoscale 13(15):7052–7080
Wang H, Huang B, Yu C, Lu M, Huang H, Zhou Y (2019) Research progress, challenges and perspectives on the sulfur and water resistance of catalysts for low temperature selective catalytic reduction of NOx by NH3. Appl Catal A 588:117207
Sun P, Cheng X, Lai Y, Wang Z, Ma C, Chang J (2018) NOx reduction by CO over ASC catalysts in a simulated rotary reactor: effect of CO2, H2O and SO2. RSC Adv 8:36604–36615
Acknowledgements
The present work was supported by the National Natural Science Foundation of China (51906193).
Funding
National Natural Science Foundation of China,51906193,denghui wang
Author information
Authors and Affiliations
Contributions
FW: Data curation, Formal analysis, Validation, Writing-original draft. YH: Supplementary experimental data. DW: Investigation, review & editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, F., Huang, Y. & Wang, D. Experimental study on supported MnO2-based catalysts for NO oxidation. Reac Kinet Mech Cat 136, 251–266 (2023). https://doi.org/10.1007/s11144-022-02343-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02343-2