Abstract
The hydrogenation and isomerization of the three isomeric propenylbenzenes, allylbenzene (AB), trans-β-methylstyrene (TBMS) and cis-β-methylstyrene (CBMS), in the liquid phase, was investigated over a 1% Pt/alumina catalyst at 313 K and 1 barg. When reacted individually, the cis-isomer gave the fastest rate of hydrogenation followed by AB, with the trans-isomer having the slowest rate giving a ratio of rates of CBMS:AB:TBMS of 57:19:1. All isomers gave high selectivity to phenylpropane, with isomerization controlled by thermodynamic constraints. Competitive hydrogenation revealed that AB adsorbed on a different site from CBMS and TBMS and that AB inhibited hydrogenation of the other two isomers. In contrast, isomerization was unaffected. These results indicate that AB adsorption limits the supply of hydrogen to the other isomers such that reductive elimination of the half-hydrogenated alkyl species to the alkane is depressed while β-elimination of the alkyl to give an olefin is not affected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is easy to assume that, as the hydrogenation of alkenes to alkanes has been researched for over 100 years, our understanding would be complete. However as Bond [1] remarks in his book “the reaction (alkene hydrogenation) is much more than the simple addition of a molecule of hydrogen or of two hydrogen atoms to the carbon–carbon double bond”. Similarly, Zaera [2] in a review article outlines the many areas that are still poorly understood. In recent years studies have shown that although the hydrogenation of ethene is structure insensitive, higher homologues such as propene [3, 4], pentene [5], 1-hexene [6] and stilbene [6] do show structure sensitivity. Not only does hydrogenation show structure sensitivity but olefin isomerization also shows sensitivity to crystal face as shown in some very elegant work by Zaera et al. [7 and references therein]. They investigated alkene isomerization and showed over Pt that the shape of the crystallite, and hence the crystal face, had a significant effect on trans–cis and cis–trans isomerization such that the rate of each reaction was different depending on the starting isomer. Another complexity is that the same reaction/study is rarely performed on more than one metal and it has been suggested [2] that there is a significant difference between palladium and the other precious metals due to its ability to absorb hydrogen. Therefore, these apparently simple reactions are complex and sensitive to surface structure.
Hydrogenation of ethene [8,9,10], propene [11, 12], butenes [13, 14], cyclohexene [6, 15, 16], 1-hexene [6] and stilbene [6] has been studied over platinum but the study of propenylbenzenes has only been undertaken over rhodium [17]. Allylbenzene (AB) hydrogenation over platinum has been reported by Cerveny [18] but only initial rate data was reported.
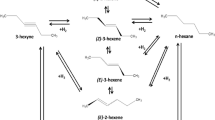
In this study we have examined the hydrogenation of alkenyl aromatics, namely AB, cis-β-methylstyrene (CBMS) and trans-β-methylstyrene (TBMS) over a Pt/alumina catalyst. These species are isomers and, in principle, give us information on how steric and electronic effects may influence hydrogenation and isomerization. As well as studying their individual hydrogenation we have also examined competitive hydrogenation where, as is often the case, two or three isomers were present in the reaction mix. The system is outlined in Fig. 1.
Experimental
Materials
The catalyst used throughout this study was a 1% w/w Pt/θ-alumina (Johnson Matthey, characterized by a BET surface area of 119 m2 g−1, a pore volume of 0.49 cm3 vg−1 and platinum dispersion of 56% giving a particle size ~ 2 nm). The Pt/alumina catalyst was pre-reduced and stabilized before being used as the temperature achievable within the reactor was not sufficiently high enough to guarantee full reduction of the catalyst in situ. Therefore, the catalyst was pre-reduced in a hydrogen stream by heating to 523 K at 10 K min−1 before maintaining the catalyst at this temperature for 2 h in flowing hydrogen (25 cm3 min−1). The flow was then switched to argon and the catalyst cooled to room temperature. To stabilize the catalyst, the gas flow was changed to 2% oxygen/argon (25 cm3 min−1) for 0.5 h before the catalyst was discharged into the air. The temperature at which re-reduction of the Pt/alumina occurred was determined by temperature-programmed reduction and was found to occur at temperatures of 333 K and above, which was accessible by the Buchi hydrogenation reactor. Allylbenzene (AB, 3-phenylprop-1-ene, Sigma-Aldrich, > 98%), cis-β-methylstyrene (CBMS, (Z)-3-phenylprop-2-ene, Sigma-Aldrich, > 95%) and trans-β-methylstyrene (TBMS, (E)-3-phenylprop-2-ene, Sigma-Aldrich, > 99%), were all used without further purification.
Hydrogenations
The reactions were carried out in a 0.5 l Buchi stirred autoclave with a hydrogen-on-demand delivery system. Around 0.05 g of catalyst was added to 330 ml of degassed solvent, 2-propanol (IPA, > 99.5%, Fisher). Reduction of the catalyst was performed in situ by sparging the system with H2 (300 cm3 min−1) for 30 min at 333 K while stirring the contents of the autoclave at 800 rpm. After reduction, the autoclave was adjusted to the appropriate reaction temperature under a nitrogen atmosphere. For AB, CBMS and TBMS 1.0 ml (AB 7.5 mmol, TBMS and CBMS 7.7 mmol) was injected into an unstirred solution, followed by 20 ml of degassed 2-propanol (IPA) to ensure that all the reactant was washed into the reactor. For the competitive reactions, 1.0 ml of each reactant was added simultaneously. The autoclave was then mixed briefly at a stirrer speed of 800 rpm and pressurized to 1 barg with N2 and a sample was taken via a sample valve. The vessel was depressurized and then pressurized with H2 to 1 barg. Following this the stirrer was set to a speed of 1000 rpm and samples taken at regular intervals.
Analyses
Liquid samples were analyzed by GC using an FID detector and a CP-Al2O3/Na2SO4 column (50 m × 0.25 mm ID, 1 μm film thickness) run at 473 K for 30 min. The stirrer speed was varied to confirm that the system was not under mass transport control, the results are shown in Fig. 2. The reaction used was hydrogenation of CBMS, which had the highest rate of reaction of all reactants used in this study.
Results
The three isomers were hydrogenated to phenylpropane (PP) at 313 K and 1 barg. The results are shown in Figs. 3, 4 and 5. It can be seen that AB & CBMS isomers rapidly hydrogenate to PP and also isomerize to TBMS. In contrast, TBMS reacts only slowly to give principally PP.
The initial rate of formation was calculated for PP in each reaction system and is reported in Table 1. An initial rate of isomerization was also calculated and reported.
The loss of the reactant(s) was analyzed using first order kinetics and a first order rate constant determined and detailed in Table 1.
The pair reactions are shown in Figs. 6, 7 and 8 and the reaction with all three isomers is shown in Fig. 9. When TBMS was one of the reactants in a competitive reaction the initial behavior was that the concentration of TBMS increased before slowly decaying.
Discussion
Typically, the more sterically hindered the C=C double bond, the harder it is to hydrogenate and hence a slower rate will be seen [19]. A typical ordering oft quoted is:

Hence it should be expected that AB would hydrogenate faster than CBMS, which would hydrogenate faster than TBMS. However the results reported reveal that CBMS has the fastest rate of hydrogenation and that on examination of the rate constants (Table 1) a ratio of CBMS:AB:TBMS of 57:19:1 is observed. Similar behaviour has been observed with hydrogenation of alkenyl aromatics over Pd [17] and Rh [17], but with less difference between the rate of hydrogenation of the isomers with ratios of CBMS:AB:TBMS of 3.8:2.2:1 and 4.2:2.8:1 respectively [17]. These results are not limited to propenylbenzenes with similar data reported for pentene hydrogenation over Pd [20], where the cis-isomer was also found to be the most reactive followed by 1-pentene while the trans-isomer was the least reactive and with butene hydrogenation [21] where the trans-isomer hydrogenated significantly slower than cis-2-butene and 1-butene. The slow rate of hydrogenation associated with trans-isomers has been attributed to strength of bonding [22, 23] and thermodynamic stability. However, a thermodynamic argument does not explain why CBMS is more reactive than AB. In a study of pentene hydrogenation over a nickel catalyst, McGregor and Gladden [23] showed that carbon deposition during pentene hydrogenation followed the sequence cis-2-pentene < 1-pentene < trans-2-pentene and that this was in keeping with the strength of adsorption. If we assume that similar behavior is occurring with the alkenyl benzenes, then the strength of adsorption would be TBMS > AB > CBMS. Indicating that the most strongly bound species was the least reactive. However, the mode of adsorption must also be considered. Typically, it is the π-adsorbed state that is involved in hydrogenation and isomerization [1, 24] and studies of butene adsorption over platinum [25] have shown that there are differences between the isomers and the mode of adsorption. Over Pt(111) the di-σ adsorbed trans-isomer was more strongly bound than the cis-isomer, whereas the π-adsorbed cis-isomer was more stable than similarly adsorbed trans-isomer. This behavior was reinforced when hydrogen was co-adsorbed [25]. Therefore, the trans isomer may be more stable and strongly bound as an inactive di-σ species while the cis isomer is more stable in the active π-adsorbed form.
This leads on to reaction selectivity. Comparing selectivity to PP at ~ 23% conversion (from Figs. 3, 4 and 5) we obtain values of 70.2% for AB, 68.5% for CBMS and 93.5% for TBMS. These high values are typical of alkene hydrogenation over platinum [13, 14], where hydrogenation is significantly favoured over isomerization. The isomerization rates are under thermodynamic control, which is reflected in the isomerization of AB and CBMS to TBMS, whereas TBMS and CBMS do not isomerize at any significant rate to AB. A low rate is also observed for TBMS isomerization to CBMS. The equilibrium isomer composition at 313 K is approximately 97% TBMS, 3% CBMS and < 0.1% AB [26], hence isomerization behavior can be explained by thermodynamic drivers.
The competitive reaction of AB and CBMS (Fig. 6) revealed a significant change in selectivity with the yield of TBMS matching that of PP. This was unexpected as in every single-isomer reaction, PP was the preferred product. The results suggested that CBMS could isomerize but not hydrogenate, whereas AB could both isomerize and hydrogenate although at slightly reduced rates. CBMS reactivity dropped and it can be shown that if we subtract the amount of TBMS produced by CBMS in the individual experiment then the amount of TBMS left is similar to that formed by AB in the solo reaction. The amount of PP produced is less than that found with the AB single experiment indicating that the reactivity of AB is also reduced. Nevertheless, the conversion of CBMS is due to isomerization to TBMS with no hydrogenation.
This is shown in Fig. 10. A similar scenario is observed with the competitive reaction between AB and TBMS; AB can isomerize and hydrogenate whereas TBMS can only isomerize however this is limited due to thermodynamic constraints giving an overall increase in TBMS until the majority of the AB is reacted. Therefore, in both reactions with AB, both CBMS and TBMS isomers adsorb on the catalyst surface and isomerize but do not hydrogenate. When all three isomers are hydrogenated simultaneously a similar phenomenon was observed; AB hydrogenated and isomerized, CBMS and TBMS only isomerized until all AB was reacted. Similar behavior was also observed over Rh/silica [17], where AB inhibited hydrogenation of CBMS and TBMS but allowed isomerization. These results imply that the adsorption/reaction site for AB is not the same as the site for CBMS and TBMS and indeed different adsorption sites for terminal and internal olefins have been proposed [5, 17, 27]. However, given that hydrogenation and isomerization have a common intermediate in the half-hydrogenated alkyl, the addition of AB inhibits reductive elimination of the alkyl (addition of hydrogen) formed from CBMS and TBMS but allows beta elimination (removal of hydrogen) to form an olefin. This suggests that AB addition affects the supply of hydrogen to CBMS and TBMS reaction sites.
Conclusions
The hydrogenation and isomerization of the three isomeric propenylbenzenes have been investigated over a Pt/alumina catalyst in the liquid phase at 313 K. The order of reactivity was CBMS > AB > TBMS, which agreed with order found over Pd and Rh [17]. All isomers gave high selectivity to PP, with isomerization controlled by thermodynamic constraints. Competitive hydrogenation revealed that AB adsorbed on a different site from CBMS and TBMS and that AB inhibited hydrogenation of the other two isomers. In contrast, isomerization was unaffected. These results indicate that AB adsorption limits the supply of hydrogen to the other isomers such that reductive elimination of the half-hydrogenated alkyl species to the alkane is depressed while β-elimination of the alkyl to give an olefin is not affected. These results show that Pt, Pd and Rh all behave in a similar manner and suggest a common mechanism for olefin hydrogenation and isomerization for all three metals.
References
Bond GC (2005) Metal-catalysed reactions of hydrocarbons. Springer, New York
Zaera F (2013) Phys Chem Chem Phys 15:11988–12003
Burwell RL Jr (1986) Langmuir 2:2–11
Otero-Schipper PO, Wachter WA, Butt JB, Cohen JB, Burwell RL Jr (1977) J Catal 50:494–507
Doyle AM, Shaikhutdinov SK, Freund H-J (2005) Angew Chem Int Ed 44:629–631
Ming Cao M, Miyabayashi K, Shen Z, Ebitani K, Miyake M (2011) J Nanopart Res 13:5147–5156
Zaera F (2009) Acc Chem Res 42:1152–1160
Dorling TA, Eastlake MJ, Moss RL (1969) J Catal 14:23–33
Zaera F (1990) J Phys Chem 94:5090–5095
Crampton A, Rötzer M, Ridge C, Schweinberger FF, Heiz U, Yoon B, Landman U (2016) Nat Commun 7:10389
Zhivonitko VV, Kovtunov KV, Beck IE, Ayupov AB, Bukhtiyarov VI, Koptyug IV (2011) J Phys Chem C 115:13386–13391
Kennedy DR, Webb G, Jackson SD, Lennon D (2004) Appl Catal A 259:109–120
Bond GC, Winterbottom JM, Phillipson JJ, Wells PB (1964) Trans Faraday Soc 60:1847–1864
Boitiaux P, Cosyns J, Robert E (1987) Appl Catal 32:145–168
Davis SM, Somorjai GA (1980) J Catal 65:78–83
Sermon PA, Georgiades G, Vong MSW, Martin-Luengo MA, Reyes PN (1987) Proc R Soc Lond A 410:353–372
Begley LC, Kakanskas KJ, Monaghan A, Jackson SD (2012) Catal Sci Technol 2:1287–1291
Kacer P, Tobicık J, Kuzma M, Cervený L (2003) J Mol Catal A 195:235–243
Rylander PN (1967) Catalytic hydrogenation over platinum metals. Academic Press Inc, London
Canning AS, Jackson SD, Monaghan A, Wright T (2006) Catal Today 116:22–29
Bond GC, Winterbottom JM (1969) Trans Faraday Soc 65:2779–2793
Canning AS, Jackson SD, Monaghan A, Spence RR, Wright T (2008). In: Prunier M (ed) Catalysis of organic reactions. Taylor & Francis, Boca Raton, pp 99–102
McGregor J, Gladden LF (2008) Appl Catal A: Gen 345:51–57
Zaera F (1996) Langmuir 12:88–94
Zaera F (2008) J Am Chem Soc 130:14924–14925
Taskinen E, Lindholm N (1994) J Phys Org Chem 7:256–258
Garcia PE, Lynch AS, Monaghan A, Jackson SD (2011) Catal Today 164:548–551
Author information
Authors and Affiliations
Contributions
AB: Validation, Formal analysis, Investigation. SDJ: Supervision, Project administration, Formal analysis, Writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bansal, A., Jackson, S.D. Hydrogenation and isomerization of propenylbenzene isomers over Pt/alumina. Reac Kinet Mech Cat 135, 1457–1468 (2022). https://doi.org/10.1007/s11144-022-02207-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02207-9