Abstract
A heterogeneous solid acid catalyst, sulfonic acid supported on pericarp-pomegranate (sulfonic acid@PP) is prepared with green an eco-friendly approach. The prepared sulfonic acid@PP catalyst was extensively characterized by IR, FE-SEM, EDX and TGA techniques. The efficiency of the catalyst has been investigated for the synthesis of bis(indolyl)alkanes by electrophilic substitution reaction of indoles with carbonyl compounds in ethanol at 80 oC. Easy recovery by simple filtration and at least three times reusability without significant loss in the yield of the desired product are conspicuous features of the reported catalyst. In addition, the notable features of this protocol are high conversions, shorter reaction times, cleaner reaction profile, simple experimental and work-up procedure.
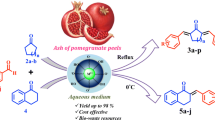
Graphic abstract











Similar content being viewed by others
References
Bell R, Carmeli S, Sar N (1994) J Nat Prod 57:1587–1590
Benabadji SH, Wen R, Zheng J, Dong X, Yuan S (2004) Acta Pharmacol Sin 25:666–671
Fahy E, Potts BCM, Faulkner DJ, Smith K (1991) J Nat Prod 54:564–569
Irie T, Kubushirs K, Suzuki K, Tsukazaki K, Umezawa K, Nozawa S (1999) Anticancer Res 31:3061–3066
Kamal A, Khan MNA, Reddy KS, Srikanth YVV, Ahmed SK, Kumar KP, Murthy US (2009) J Enzym Inhib Med Chem 24:559–565
Kobayashi M, Aoki S, Gato K, Matsunami K, Kurosu M, Kitagawa I (1984) Chem Pharm Bull 42:2449–2451
Kuethe JT (2006) Chimia 60:543–553
Shiri M, Zolfigol MA, Kruger HG, Tanbakouchian Z (2010) Chem Rev 110:2250–2293
Sivaprasad G, Perumal PT, Prabavathy VR, Mathivanan N (2006) Bioorg Med Chem Lett 16:6302–6305
Sujatha K, Perumal PT, Muralidharan D, Rajendran M (2009) Indian J Chem 48B:267–272
Zeligs MA (1998) J Med Food 1:67–70
Wang Y, Sang R, Zheng Y, Guo L, Guan M, Wu Y (2017) Catal Commun 89:138–142
Nagarajan R, Perumal PT (2002) Tetrahedron 58:1229–1232
Bandgar BP, Shaikh KA (2003) Tetrahedron Lett 44:1959–1961
Yadav JS, Reddy BVS, Murthy VSR, Kumar GM, Kumar M (2001) Synthesis 5:783–787
Ghorbani-Vaghei R, Veisi H, Keypour H, Dehghani-Firouzabadi AA (2010) Mol Divers 14:87–97
Nagarajan R, Perumal PT (2004) Chem Lett 3:288–289
Singh PR, Singh DU, Samant SD (2005) Synth Commun 35:2133–2138
Siadatifard SH, Abdoli-Senejani M, Bodaghifard MA (2016) Cogent Chem 2:1188435–1188441
Sharma GVM, Reddy JJ, Lakshmi PS, Krishna PR (2004) Tetrahedron Lett 45:7729–7732
Merinosa JPG, Ruiza HL, Lopez Y, Limaa SR (2015) Lett Org Chem 12:332–336
Magesh CJ, Nagarajan R, Karthik M, Perumal PT (2004) Appl Catal A Gen 266:1–10
Karthik M, Tripathi AK, Gupta NM, Palanichamy M, Murugesan V (2004) Catal Commun 5:371–375
Hosseini-Sarvari M (2008) Synth Commun 38:832–840
Harichandran G, Amalraj SD, Shanmugam P (2016) J Saudi Chem Soc 22:208–217
Babu G, Sridhar N, Perumal PT (2000) Synth Commun 30:1609–1614
Satam JR, Parghi KD, Jayaram RV (2008) Catal Commun 9:1071–1078
Veisi H, Sedrpoushan A, Zolfigol MA, Mohanazadeh F (2011) J Heterocyclic Chem 48:1448–1454
Reddy BM, Sreekanth PM, Lakshmanan P (2005) J Mol Catal A 237:93–100
Meshram GA, Patil VD (2010) Synth Commun 40:29–38
Yadav JS, Reddy BVS, Sunitha S (2003) Adv Synth Catal 345:349–352
Mi X, Luo S, He J (2004) C Jin-Pei. Tetrahedron Lett 45:4567–4571
Mo LP, Ma ZC, Zhang ZH (2005) Synth Commun 35:1997–2004
Nguyen HTD, Nguyen TT, Nguyen PTK, Tran PH (2020) Arab J Chem 13:1377–1385
Pokhre R, Ghosh D, Jha S, Bhattacharyya NK, Jha S (2014) Int J Innov Res Sci Eng Tech 3:15666–15671
Deb ML, Deka B, Saikia PJ, Baruah PK (2017) Tetrahedron Lett 58:1999–2003
Sobhani S, Jahanshahi R (2013) New J Chem 37:1009–1015
Reddy MK, Gupta SK, Jacob MR, Khan SI, Ferreira D (2007) Planta Med 73:461–467
Kotwal GJ (2008) Vaccine 26:3055–3058
Bell C, Hawthorne S (2008) J Pharm Pharmacol 60:139–144
Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, Heber D (2005) J Nutr Biochem 16:360–367
Negi PS, Jayaprakash GK, Jena BS (2003) Food Chem 80:393–397
Seeram N, Lee R, Hardy M, Heber D (2005) Sep Purif Technol 41:49–55
Amin NK (2009) J Hazard Mater 165:52–62
Bhatnagar A, Minocha AK (2009) J Hazard Mater 168:1111–1116
El-Ashtoukhya ESZ, Amin NK, Abdelwahab O (2008) Desalination 223:162–173
El Nemr A (2007) Chem Ecol 23:409–425
El Nemr A (2009) J Hazard Mater 161:132–141
Bhatnagar A, Minocha AK (2010) Colloids Surf B Biointerfaces 76:544–548
Rohani Moghadam M, Nasirizadeh N, Dashti Z, Babanezhad E (2013) Int J Ind Chem 4:19–24
Sadeghi B, Bouslik M, Shishehbore MR (2015) J Iran Chem Soc 12:1801–1808
Tayebee R, Amini MM, Nehzat F, Sadeghi O, Armaghan M (2013) J Mol Catal A 366:140–148
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, M., Karhale, S., Bhenki, C. et al. Sulfonic acid@pericarp-pomegranate: A natural supported catalyst for synthesis of bis(indolyl)alkanes. Reac Kinet Mech Cat 130, 993–1007 (2020). https://doi.org/10.1007/s11144-020-01828-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01828-2