Abstract
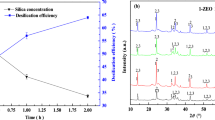
Desilication in the solution with Na2O concentration below 300 g/L was extensively studied, such as various desilication agents mostly of calcium salts or compounds proposed in the literature. However, desilication in high-alkali sodium aluminate solution was necessary in the production of sodium aluminate hydrate (SAH), but much difficult as the high silicate solubility in concentrated caustic solution. Therefore, an effective desilication method was investigated with different mechanism in this paper. The hydroxy-sodalite compound was synthesized and found with excellent desilication properties in highly caustic aluminate solution until 470–530 g/L Na2O, and the desilication mechanism in concentrated sodium aluminate solution was presented firstly. The crystallization reaction of silicate with aluminate in the sodium aluminate solution during the desilication was proposed. It was observed that the aluminate concentration in the concentrated solution considerably affected the desilication. Furthermore, the kinetics equation was inferred as − dσ/dt = exp(29.30–11311/T) (σ)n, with the obtained apparent activation energy and order dependence of 94.04 kJ/mol and 2.47 ± 0.47. Meanwhile, the equilibrium SiO2 concentrations of the synthesized hydroxy-sodalites in the sodium aluminate solutions with 470–530 g/L Na2O at 353–393 K were determined.










Similar content being viewed by others
References
Milacic R, Zuliani T, Scancar J (2012) Environmental impact of toxic elements in red mud studied by fractionation and speciation procedures. Sci Total Environ 426:359–365. https://doi.org/10.1016/j.scitotenv.2012.03.080
Liu Y, Lin C-X, Wu Y-G (2007) Characterization of red mud derived from a combined Bayer process and bauxite calcination method. J Hazard Mater 146(1–2):255–261. https://doi.org/10.1016/j.jhazmat.2006.12.015
Zhu DQ, Chun TJ, Pan J, He Z (2012) Recovery of iron from high-iron red mud by reduction roasting with adding sodium salt. J Iron Steel Res Int 19(8):1–5
Liu DY, Wu CS (2012) Stockpiling and comprehensive utilization of red mud research progress. Materials 5(7):1232–1246. https://doi.org/10.3390/ma5071232
Nikolaev IV, Kirov SS, Vorob’ev IB, Zakharova VI, Bogatyrev BA, Magazina LO (2011) Applicability of hydrogarnet technology for complex processing of Indian condalites. Russ J Non-Ferr Met 52(2):150–156. https://doi.org/10.3103/s106782121102009x
Ma SH, Wen ZG, Chen JN, Zheng SL (2009) An environmentally friendly design for low-grade diasporic-bauxite processing. Miner Eng 22(9–10):793–798. https://doi.org/10.1016/j.mineng.2009.02.007
Rayzman V, Filipovich I, Nisse L, Vlasenko Y (1998) Sodium aluminate from alumina-bearing intermediates and wastes. JOM 50(11):32–37. https://doi.org/10.1007/s11837-998-0284-8
Mon J, Deng Y-J, Flury M, Harsh JB (2005) Cesium incorporation and diffusion in cancrinite, sodalite, zeolite, and allophane. Microporous Mesoporous Mater 86(1–3):277–286. https://doi.org/10.1016/j.micromeso.2005.07.030
Lin D-C, Xu X-W, Zuo F, Long Y-C (2004) Crystallization of JBW, CAN, SOD and ABW type zeolite from transformation of meta-kaolin. Microporous Mesoporous Mater 70(1–3):63–70. https://doi.org/10.1016/j.micromeso.2004.03.003
Walton RI, O’hare D (2001) An in situ energy-dispersive X-ray diffraction study of the hydrothermal crystallization of zeolite A. 2. Effect of deuteration on crystallization kinetics. J Phys Chem B 105 (1):91–96. https://doi.org/10.1021/jp002712h
Smith P (2009) The processing of high silica bauxites—review of existing and potential processes. Hydrometallurgy 98(1–2):162–176. https://doi.org/10.1016/j.hydromet.2009.04.015
Liu W-C, Yang J-K, Xiao B (2009) Review on treatment and utilization of bauxite residues in China. Int J Miner Process 93(3–4):220–231. https://doi.org/10.1016/j.minpro.2009.08.005
You SW, Zhang YF, Cao ST, Chen FF, Zhang Y (2012) Crystallization of monosodium aluminate hydrate from a concentrated aluminate solution containing silica. Hydrometallurgy 115:104–107. https://doi.org/10.1016/j.hydromet.2012.01.001
Zheng K, Gerson AR, AddaiMensah J, Smart RS (1997) The influence of sodium carbonate on sodium aluminosilicate crystallisation and solubility in sodium aluminate solutions. J Cryst Growth 171(1–2):197–208. https://doi.org/10.1016/S0022-0248(96)00480-0
Barnes MC, Addai-Mensah J, Gerson AR (1999) The kinetics of desilication of synthetic spent Bayer liquor seeded with cancrinite and cancrinite/sodalite mixed-phase crystals. J Cryst Growth 200(1–2):251–264. https://doi.org/10.1016/S0022-0248(98)01294-9
Ma J-Y, Zhai K-M, Li Z-B (2011) Desilication of synthetic Bayer liquor with calcium sulfate dihydrate: kinetics and modelling. Hydrometallurgy 107(1–2):48–55. https://doi.org/10.1016/j.hydromet.2011.01.002
Zheng K, Smart RS, Addai-Mensah J, Gerson A (1998) Solubility of sodium aluminosilicates in synthetic Bayer liquor. J Chem Eng Data 43(3):312–317. https://doi.org/10.1021/Je970187i
Whittington BI (1996) The chemistry of CaO and Ca(OH)(2) relating to the Bayer process. Hydrometallurgy 43(1–3):13–35. https://doi.org/10.1016/0304-386x(96)00009-6
Ma J-Y, Li Z-B, Zhang Y, Demopoulos GP (2009) Desilication of sodium aluminate solution by Friedel’s salt (FS: 3CaO center dot Al2O3 center dot CaCl2 center dot 10H(2)O). Hydrometallurgy 99(3–4):225–230. https://doi.org/10.1016/j.hydromet.2009.08.010
J-l Yuan, Zhang Y (2009) Desiliconization reaction in sodium aluminate solution by adding tricalcium hydroaluminate. Hydrometallurgy 95(1–2):166–169. https://doi.org/10.1016/j.hydromet.2008.02.011
Zeng LM, Li ZB (2012) Solubility and Modeling of Sodium Aluminosilicate in NaOH-NaAI(OH)(4) Solutions and Its Application to Desilication. Ind Eng Chem Res 51(46):15193–15206. https://doi.org/10.1021/ie301590r
L-n Shi, Ruan S, Li J, Gerson AR (2017) Desilication of low alumina to caustic liquor seeded with sodalite or cancrinite. Hydrometallurgy 170:5–15. https://doi.org/10.1016/j.hydromet.2016.06.023
Jamialahmadi M, Muller-Steinhagen H (1998) Determining silica solubility in Bayer process liquor. JOM 50(11):44–49
Murakami T, Sugano Y, Kinami T, Narushima T, Iguchi Y, Ouchi C (2011) Alkali hydrothermal synthesis of zeolite A using oxide by-products. ISIJ Int 51(1):158–165. https://doi.org/10.2355/isijinternational.51.158
Shoumkova A, Stoyanova V (2013) Zeolites formation by hydrothermal alkali activation of coal fly ash from thermal power station “Maritsa 3’’, Bulgaria. Fuel 103:533–541. https://doi.org/10.1016/j.fuel.2012.07.076
Chen F-F, Zhang Y-F, Jiang X-D, Cao S-T, You S-W, Zhang Y (2016) Structure transformation of sodium aluminosilicates as desilication agents in the desilication of highly alkaline sodium aluminate solution containing silica. Microporous Mesoporous Mater 235:224–232. https://doi.org/10.1016/j.micromeso.2016.08.011
Lente G (2015) Deterministic kinetics in chemistry and systems biology. Springer, New York. https://doi.org/10.1007/978-3-319-15482-4
Barnes MC, Addai-Mensah J, Gerson AR (1999) The kinetics of desilication of synthetic spent Bayer liquor and sodalite crystal growth. Colloid Surface A 147(3):283–295. https://doi.org/10.1016/S0927-7757(98)00570-6
Noworyta A (1981) On the removal of silica from aluminate solutions - mechanism and kinetics of the process. Hydrometallurgy 7(1–2):99–106. https://doi.org/10.1016/0304-386x(81)90015-3
Gualtieri A, Norby P, Artioli G, Hanson J (1997) Kinetic study of hydroxysodalite formation from natural kaolinites by time-resolved synchrotron powder diffraction. Microporous Mater 9(3–4):189–201. https://doi.org/10.1016/S0927-6513(96)00111-3
Acknowledgements
This work is supported by the National High Technology Research and Development Program of China (863 Program, No. 2011AA060701).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, X., Zhang, Y. & Zhang, Y. Desilication mechanism and kinetics of synthesized hydroxy-sodalite in high-alkali sodium aluminate solutions. Reac Kinet Mech Cat 127, 489–504 (2019). https://doi.org/10.1007/s11144-019-01565-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-019-01565-1