Abstract
The measurements of the minimum acid strength (HOmin) required for the initiation of cis-stilbene at 303 K and tert-butylbenzene at 313 K, 323 K and 333 K have enabled the establishment of a much more precise acid strength scale based on test reactions. HOmin values obtained were: − 9.0 (cis-stilbene, 303 K), − 9.3, − 9.5 and − 9.9 (tert-butylbenzene, 313 K, 323 K, 333 K). The updated method was used for the determination of the acid strength of different catalysts obtained in the reaction of chlorosulfonic and sulfuric acid on different oxide carriers. It was found that the acid strength values depended on the basicity of the studied supports. For the supports treated with chlorosulfonic acid the values of acid strength were: − 11.5 < HO ≤ − 10.8 in the case of the less basic SiO2 and − 9.3 < HO ≤ − 9.0 for the more basic TiO2, Al2O3 and MgO. For the supports treated with sulfuric acid the acid strength was: -9.3 < HO ≤ -9.0 for the less basic SiO2 and TiO2, − 9.0 < HO ≤ − 7.9 for the more basic Al2O3 and − 7.9 < HO ≤ − 6.9 for the most basic MgO. Silica treated with chlorosulfonic acid possesses higher acid strength than HZSM-5 and amorphous silicaalumina. The catalyst obtained in the modification of TiO2, Al2O3 and MgO with chlorosulfonic acid were of lower acid strength comparable to Amberlyst 15 and HZSM-5 (− 9.3 < HO ≤ − 9.0), but higher than p-xylene-2-sulfonic acid dihydrate (− 7.9 < HO ≤ − 6.9).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sulfuric acid is a widely used catalyst in a number of chemical reactions and technological processes [1]. The H2SO4–H2O system is inexpensive and enables the application of a wide range of acid strength up to HO = − 12. To facilitate practical applications, attempts have been made to immobilize it on solid supports. The basic method of preparing such systems is the reaction of the support with chlorosulfonic or sulfuric acid.
Support-chlorosulfonic acid system
Chlorosulfonic acid possesses a high acid strength (HO = − 13.8) [2], and is also characterized by the presence of chemically active sulfur-chlorine bond. This is why it readily reacts with compounds with a hydroxyl group to form corresponding esters and hydrogen chloride.
Here R denotes both an aliphatic chain and an aromatic ring [3,4,5].
This property of chlorosulfonic acid is being used for the synthesis of a whole series of solid acids in the reaction of the surface hydroxyl groups of various supports with ClSO3H. The reaction is carried out in a solvent, most often dichloromethane:
The following materials were used as supports: SiO2 [6], Al2O3 [7], TiO2 [8,9,10,11], zeolites: HZSM-5 [12], BEA [13], clays: montmorillonite [14], aluminosilicates [15] or oxide systems with oxidizing-reducing properties as TiO2–Fe2O3 [16]. The synthesized catalysts were then used in organic synthesis as alternatives to classical cationic resins such as Ambetyst 15. It is worth noting that the thermal degradation of the sulfonated supports starts at temperatures higher than for the Amberlyst 15 resin (393 K) [17]. These temperatures are 423 K for sulfonated: silica [6] and titania [18], 433 K for zeolite BEA [13] or 513 K for montmorillonite [14]. This creates the advantage in using these systems to catalyze reactions requiring higher temperatures than 393 K [9].
Support-sulfuric acid system
Sulfuric acid (HO = − 12) is also often used in the synthesis of sulfonated oxides [19]. In the absence of the weak S-Cl bond, as in the chlorosulfonic acid molecule, the mechanism of interaction with the surface of the support is somehow different. The H2SO4 used in the aqueous solution is practically completely dissociated, so the substrates, which react with the surface of the support are sulfate, hydrogen sulfate and H3O+ ions. If the basicity of a support is high enough, as for example in the case of ZrO2, then the dissociation of H2SO4 can occur on its surface even without presence of a solvent. Therefore, surface sulfate (Zr–O)3S=O, pyrosulfate (Zr–O)2S (=O)–O–S (=O) (Zr–O)2 and hydrogen sulfate (Zr–O)3 S (=O)–OH groups are formed as a result of the reaction of sulfuric acid with ZrO2, even at room temperature [20]. The proton located at the O–Zr–O bridge of the sulfate (Zr–O)3S=O group is responsible for the strongest acidic properties of sulfated zirconia system [21]. For supports with low basicity such as SiO2, sulfuric acid reacts in a similar way as in the case of alcohols [22]:
To form hydrogen sulfate moieties on the surface that are stable up to 523 K, which are converted to sulfate groups during calcination above 623 K [23, 24].
Acid properties of support-SO3H system
There are no consistent measurements made up to date to determine unambiguously the acid strength of the solid catalysts with the sulfonic and sulfate groups deposited on their surfaces. The acid strength of the resins Amberlyst 15 and Nafion, for example, was found to be − 2.2, − 9.5 HO units for Amberlyst [17, 25] and − 11 to − 13 HO units for Nafion [2, 25]. The acidity of other systems of this type, such as SiO2–CF2–CF2–SO3H, SiO2–CH2–CH2–CH2–SO3H or SiO2–CH2–CH2–Ph–SO3H, was determined by other methods that are less comparable than the use of the HO scale. In comparing the rate of α-methylstyrene dimerization reactions, it was found that the system, in which the sulfonic group was attached to the support through the perfluorinated alkyl chain was 1000 times more active than Nafion NR50 and Amberlyst 15 [26]. Based on the results of 31PNMR research on the adsorption of triethylphosphine oxide (TEPO), and the chemical shift of 31P, it can be confirmed that the acid strength of systems containing differently bound sulfonic group changes in the following sequence [27]:
SiO2 (57.9 ppm) < Al-MCM-41 (66.8 ppm) < SiO2–CH2–CH2–CH2–SO3H (71.1 ppm) < SiO2–CH2–CH2–Ph2–SO3H (78.5 ppm) < Amberlyst 15 (86.0 ppm).
It can be observed that this is between the acid strength of Al-MCM-41 zeolite and Amberlyst 15 resin. On the other hand, ammonia adsorption studies have shown that the acid strength of SiO2–CH2–CH2–CH2–SO3H is higher than that of Amberlyst 15 and lower than that of Nafion NR50 [28]:
Amberlyst 15 (110 kJ mol−1) < SiO2–CH2–CH2–CH2–SO3H (126 kJ mol−1) < Nafion NR50 (158 kJ mol−1).
The values in brackets represent the average value of molar enthalpy of NH3 adsorption.
Studies on the acidity of silica [15] and titania [18] treated with ClSO3H led also to unexpected results. Their acid strength was found to be low and amounted to 0.65 and 1.12 HO units. In comparison, the acid strength of the catalyst obtained in the reaction of H2SO4 (95%) with SiO2 was comparable to that of the sulfuric acid used [29] (approximately − 9.7 HO units).
The observed discrepancies in the assessment of the acid strength of catalysts with –SO3H groups on their surfaces prompted us to undertake systematic studies on the acidity of these systems. The materials under study were systems obtained as a result of the reaction of chlorosulfonic acid with the following supports: SiO2, Al2O3, TiO2 and MgO. The selection of such supports with very different acid–base properties should enable the determination of the influence of the basicity of the support on the acid strength of the –SO3H group. For comparison purposes, catalysts prepared with sulfuric acid instead of chlorosulfonic acid as well as typical solid acids possessing sulfonic groups such as Amberlyst 15 and p-xylene-2-sulfonic acid dihydrate were also investigated. These studies were aimed at comparing the acid strength of the SO3H group with the sulfate moieties present in H2SO4-support systems.
Acid strength measurements. The test reaction method
There are many methods used for studying acid–base properties of solids. The main are based on: probe molecules temperature programmed desorption (TPD), microcalorimetry techniques, FTIR, MAS NMR, ESR measurements. Their complete description can be found in a detailed critical review presented by Vedrine [30]. The results obtained using these methods are reliable and consistent within the method applied. The problem arises, as it was shown above for the case of support-SO3H catalysts, when one would like to compare the parameters reflecting acid strength measured by different techniques and relate them to the well known mineral acid solutions. In this study we used the test reaction method which base on Hammett acidity scale.
It is well known that the Hammett indicator adsorption method cannot be used to obtain reliable values of acid strength of solids [30, 31]. The most important reason is the fact that solid acids do not belong to the family of Hammett acids. However, this statement does not exclude the use of Hammett acidity function (HO) as a measure of acid strength of such materials. In many valuable publications one can find how one can measure (indirectly) values of Ho for solids.
For example, sulfuric acid–water solutions of known composition and then defined acid strength (HO) were used to calibrate
-
Hammett indicators UV bands shift against Ho and applied to study acidity of solids [32, 33].
-
Probe molecules (acetone, mesitil oxide) chemical shift of NMR signals against HO and applied to study acidity of solids [34,35,36].
The catalysts of known acid strength (HO) [32] were used to calibrate:
-
IR band shift of adsorbed CO against HO and applied to study acidity of solids [37].
-
Isobutane conversion against HO and applied to study acidity of zeolites [32].
In another group of works, the results of test reactions and heat of ammonia adsorption were related directly to the acid strength of catalysts. For example, the Ho function was derived using:
-
Isobutane isomerization reaction as Hammett indicator [38]. The following formula was obtained:
$$H_{O} = \frac{{E_{a} }}{{2.3RT_{1/2} }} - 22.4$$Here Ea and T1/2 are the apparent activation energy and temperature at 0.5% conversion of isobutane.
-
Ammonia, in TPD experiments, as Hammett indicator [39]. The following formula was obtained:
$$H_{O} = - 1.75*10^{ - 4} *\Delta H^{O} + 15.9$$Here with ∆HO denotes the heat of ammonia adsorption.
In our works we used the similar strategy for acid and basic strength measurements [40, 41]. It is close to the Hammett indicator method where instead of color change observation, the chemical transformation of protonated or deprotonated molecules is measured. The method based on the results of test reactions of α-methylstyrene, styrene, 2,4-diphenyl-4-methyl-1-pentene, 4-phenyl-1-butene, tert butyl benzene and cumene for acidity measurements and diacetone alcohol to acetone decomposition as well as benzaldehyde Cannizzaro reaction for basicity evaluation.
In the presence of acid catalysts alkenes and alkylaromatics undergo numerous transformations i.e. isomerization, oligomerization and disproportionation reactions. All reactions begin with the protonation of the reacting molecule. This reaction step can take place only if the acid strength of the catalyst is high enough to assure proton transfer from a catalyst to the reactant. It was demonstrated [40] that it is possible to find the minimal acid strength of a catalyst (Homin) at which a particular test reaction begins to proceed at given conditions (catalyst: 0.5 g, reagent: 2 cm3, batch reactor, temperature 303 K, reaction time 24 h). A prolonged reaction time (24 h) assured that even if a reaction rate is low or diffusion controlled, the reaction products can be observed. The calibration of the method was performed using the catalysts of strictly defined acid strength. These were aqueous solutions of sulfuric acid of various concentrations introduced into the pores of an inert carrier (SiO2). In this way, it was possible to determine the acid strength at which the reaction is initiated and substrates converted to products. The measurement of the acid strength of the solid acid (a catalyst) involve the carrying out of a series of test reactions under the calibration conditions of the method and determining which of them proceeded or not.
The values of HOmin for different substrates of the test reactions have already been determined [40] and the HO scale based on them is presented below (Fig. 1)
HO scale based on test reactions [40]
There are some ranges in the scale without designated HOmin values, such as, for example, the range between − 7.9 and − 10.8 Hammett scale units. Therefore, additional research was undertaken in the work on the possibility of using cis-stilbene (under test conditions) and tert-butylbenzene at elevated temperatures (HOmin = − 10.8 under the test conditions) to complete the scale. Both compounds react in the presence of acids. Cis-stilbene undergoes isomerization to trans-stilbene [42] and condensation to tetraline derivatives [43] while tert-butylbenzene transforms into benzene and di-tert-butylbenzenes [40]. The reaction of tert-butylbenzene was carried out by changing the temperature from 303 K to 313 K, 323 K and 333 K, while the cis-stilbene conversions were tested under standard test conditions (303 K) in the presence of catalysts of known acid strength. These were solutions of sulfuric acid introduced into the pore system of silica by dry impregnation [40]. The acid strength of the solutions was determined based on the data gathered in the work [44] assuming that changes in HO values due to temperature alterations are to be neglected [45]. We expected that an increase in the reaction temperature will lower the minimum acid strength at which tert-butylbenzene initially undergoes protonation and, then the disproportionation to benzene and di-tert-butylbenzenes [46]. The determination of new HOmin values will enable increasing the level of accuracy of the measurement of the minimum acid strength by means of the test reactions method.
Experimental
Catalysts and supports
p-Xylene-2-sulfonic acid dihydrate (BDH), Amberlyst 15 (Sigma-Aldrich), amorphous silicaalumina containing 45% Al2O3 (Pierce Inorganics, SBET = 110 m2 g−1, HZSM-5 (IChP Warsaw, SiO2/Al2O3 = 40), four oxides: SiO2 (ABCR Karlsruhe, SBET = 266 m2 g−1), ɣ-Al2O3 (Pierce Inorganics, SBET = 98 m2 g−1), TiO2 (Degussa P-25, SBET = 53 m2 g−1) and MgO (Ventron, SBET = 100 m2 g−1) of grain diameter of 1.02–1.20 mm were used as catalysts and supports.
The synthesis of catalysts with known acid strength. SiO2 (0.125 g) was placed in the vial equipped with a screw closure and impregnated by the incipient wetness method with an acid of known acid strength (0.25 cm3). The use of specific H2SO4–H2O solutions enabled the preparation of catalysts with acid strength varying from − 8 to − 12 HO units [40].
The synthesis of sulfated catalysts. 10 g of SiO2, TiO2, Al2O3 or MgO was initially dried at 423 K for 0.5 h under reduced pressure in a 100 cm3 three-neck flask equipped with a magnetic stirrer, a dropping funnel containing 3.88 g of neat chlorosulfonic acid and a joint connection to link the system with a dry N2/vacuum line. After drying the oxide, the flask was cooled to ambient temperature and in the flow of nitrogen a tube for evolving HCl leading to a trap filled with Na2CO3 water solution was connected. 50 cm3 of dichloromethane was added to the system and chlorosulfonic acid was dosed dropwise under stirring over a period of 30 min. Stirring was continued for another 30–40 min. after the addition of chlorosulfonic acid until HCl evolution stopped. Then the solvent was removed by distillation and the obtained catalyst was stored in a desiccator.
Measurements of HOmin values for test reaction substrates
2 cm3 of cis-stilbene or tert-butylbenzene was added to a catalyst of known acid strength in a vial equipped with a screw closure. The closed vial was placed in the heat chamber equipped with shaker (Mini Incubator 4010, GLF; shaking: 60 rpm, Shaker DOS-20S Elmi Ltd). After 24 h, the organic phase was separated, neutralized (three times) with 5% NaHCO3 solution and then analyzed. The minimum acid strength (HOmin) was determined according to the procedure proposed in the previous work [40]. The dependence of the total conversion of tert-butylbenzene and cis-stilbene as a function of the acid strength (HO) of catalyst was determined and then interpolated using the standard β-spline function. From the obtained relation, the acid strength value was determined as the point, at which the reaction products began to appear and conversion reached approximately 0.75% [40].
Acid strength measurements
0.5 g of the sulfated catalyst was placed in a vial fitted with a screw closure and then 2 cm3 of the appropriate substrate for the test reaction was injected. These were: 30% heptane solutions of: α-methylstyrene (HOmin = − 3.3), styrene (HOmin = − 5.3), 2,4-diphenyl-4- methyl-1-pentene (HOmin = − 6.9), 4-phenyl-1-butene (HOmin = − 7.9), 10% toluene solution of cis-stilbene as well as tert-butylbenzene (HOmin = − 10.8) and cumene (HOmin = − 11.5). The minimum acid strength values written in brackets refer to the values at reaction temperature of 303 K. The closed vials were then placed in a suitable thermal chamber at chosen temperature for 24 h. Further procedure was done according to the technique described above for determining the minimum acid strength. The reactions with tert-butylbenzene were carried out also at 313, 323 and 333 K. The acid strength of p-xylene-2-sulfonic acid dihydrate was determined in the same manner. In this case 1.0 M NaOH solution was used to neutralize the sample. The neutralization step was omitted for reactions with Amberlyst 15.
When measuring the acid strength of pure oxides, silicaalumina and HZMS-5 zeolite the samples (0.5 g) for measurement were calcined in a stream of dry air (753 K, 3 h). After cooling, the samples were transferred in an atmosphere of dry air to vials with a sealed closure and the test substrate (2 cm3) was introduced. Further procedure was as in the method described above for determining the acid strength of Amberlyst 15 resin.
Basic strength measurements
Samples of the selected oxides: SiO2, TiO2, Al2O3 and MgO were prepared in a similar manner for acid strength measurements. The difference in the method, however was that instead of the substrate, appropriate (0.1%) toluene indicator solutions were introduced to the vials. The following indicators were used in the measurements: bromothymol blue pKa = 7.2, phenolphthalein pKa = 9.3, 2,4,6-trinitroaniline pKa = 12.0, 2,4-dinitroaniline pKa = 15.0, 4-chloro-2-nitroaniline pKa = 17.2, 4-nitroaniline pKa = 26.5, triphenylmethane pKa = 33.0 [47].
Materials
All organic reactants, chlorosulfonic acid, sulfuric acid, NaHCO3, Na2CO3 and NaOH were supplied by Aldrich.
Analysis
The obtained reaction products were analyzed in a GC (Agilent 6890 N with FID detector) equipped with a 30 m HP5 capillary column (I.D. 0.32 mm, df 0.25 μm, temperature 343 K (5 min), to 543 K at 3 K/min).
Substrate conversion was calculated in two ways. It was obtained directly from GC analysis and was evaluated on the basis of changes of substrate to solvent (heptane or toluene), an inert compound serving as an internal standard, ratio. In the second way, the total conversion was calculated according to the following formula:
Here \(X_{total}\) denotes total substrate conversion, \(\beta_{substr.}^{o} = \frac{{S_{substr.}^{o} }}{{S_{stand.}^{o} }},\beta_{substr.}^{\tau } = \frac{{S_{substr.}^{\tau } }}{{S_{stand.}^{\tau } }}\), are ratios of the areas of GC peaks for substrate and internal standard (at the beginning of the reaction and at a reaction time equal to τ.
Results
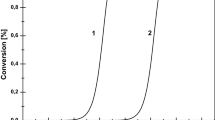
The first step of the work was the extension of the HO scale based on test reactions in the range between − 7.9 and − 10.8 units. Increasing the scale accuracy can be done in two ways. The first of these is the use of an entirely new substrate that would react in the aforementioned acid strength range. Cis-stilbene was chosen as such a reactant. On the other hand, it would seem that a more efficient procedure would be to use a known substrate but at a higher than standard (303 K) reaction temperature. The results of cis-stilbene transformation are presented below (Fig. 2).
Experimental points were connected with a β-spline type line using Origin Pro software (OriginLab Corp.) which enabled the reading of the value of conversion for any Ho value. According to the procedure adopted in the previous work [40], it was assumed that the reaction starts at the point when the conversion rate exceeds 0.75%. In this way HOmin value was obtained. This was found to be − 9.0 units. The second method that was used for improving the acid strength scale was performing test reaction at higher temperatures. The tert-butylbenzene test reaction at 303 K starts when acid strength of a catalyst reaches − 10.8 units on the Ho scale [30]. It can be assumed that at elevated temperatures this reaction should, according to the Arrhenius Law, take place at a quicker rate and thus at lower acid strength values. To test this assumption, tert-butylbenzene reactions were carried out at three temperatures (313 K, 323 K and 333 K) in the presence of catalysts with known acid strengths of − 9.0; − 9.4; − 9.8; − 11.0 and − 12.0 Ho scale units. Benzene and di-tert-butylbenzenes were obtained as products confirming the fact that that acid catalyzed transalkylation reaction actually took place. The results are presented below (Fig. 3).
Dependence of tert-butylbenzene conversion at 333 K (filled inverted triangle), 323 K (filled triangle), 313 K (filled circle) and 303 K (filled sqaure) on acid strength of catalyst. Batch reactor, catalyst: SiO2 (0.125 g)—H2SO4–H2O (0.25 cm3), substrate: 2.00 cm3. Dotted line indicates arbitrary taken level of the conversion at which reaction begin to proceeds
HOmin values for tert-butylbenzene were determined using the same procedure as in the case of cis-stilbene. They were − 9.3, − 9.5 and − 9.9 units for 313 K, 323 K and 333 K. The newly obtained HOmin values were inserted into the expected range of − 10.8 < Ho < − 9.0 units thus making the acid strength scale more precise. The complete set of test reaction substrates, i.e. α-methylstyrene, styrene, 2,4-diphenyl-4-methyl-1-pentene, 4-phenyl-1-butene, cis-stilbene, tert-butylbenzene and cumene, was then used to examine the acid strength of selected catalysts in the reactions that were carried out at 303 K and in the case of tert-butylbenzene additionally at 313 K, 323 K and 333 K. First, the unmodified systems (simple oxides: alumina, titania, silica, magnesia), mixed oxides: SiO2–Al2O3 (45%), HZSM-5 zeolite with Si/Al ratio of 40 as well as p-xylene-2-sulfonic acid dihydrate and Amberlyst 15 were examined (Table 1).
The silicon, aluminum and magnesium oxides did not catalyze any test reaction, so their acid strength is Ho > − 3.3. Titanium oxide catalyzed only the transformation of α-methylstyrene (oligomerization). The conversion of styrene to traces of dimers (0.1%) does not confirm that this test reaction actually took place. Thus, the acid strength of TiO2 is − 5.3 < Ho ≤ − 3.3. p-Xylene-2-sulfonic acid dihydrate initiates the reaction of two substrates, namely α-methylstyrene (oligomerization) and styrene (oligomerization), indicating that its acid strength is in the range − 6.9 < Ho ≤ − 5.3. The conversion of 0.5% observed for the reaction of 2,4-diphenyl-4-methyl-1-pentene (isomerization and oligomerization) is lower than the value adopted for the determination of HOmin, so it does not confirm the course of this test reaction. Amberlyst 15 and HZSM-5 zeolite catalyzed the oligomerization of α-methylstyrene and styrene, isomerization and oligomerization of: 2,4-diphenyl-4-methyl-1-pentene, 4-phenyl-1-benzene and cis-stilbene. Thus, all systems have a similar acid strength of − 9.3 < HO ≤ − 9.0. Amorphous silicaalumina SiO2–Al2O3(45%) additionally initiated tert-butylbenzene transalkylation at 333 K, thus it possesses the highest acid strength of − 9.5 < HO ≤ − 9.3. The results of the acid strength measurements for the catalysts obtained by reaction of the support with chlorosulfonic and sulfuric acids were collected in Table 2.
The introduction of the SO3H group on the surface of the support in the reaction with chlorosulfonic acid results in the formation of catalysts with high acid strength. For SiO2–ClSO3H catalyst, the acid strength increased to − 10.8 < HO ≤ -9.9 while for the remaining oxides (Al2O3, TiO2, MgO) treated with chlorosulfonic acid the value was lower: − 9.3 < HO ≤ − 9.0. The interaction of sulfuric acid with the supports leads to the catalysts with the following acid strength: for SiO2–H2SO4 and TiO2–H2SO4 it was − 9.3 < HO ≤ − 9.0 while for Al2O3–H2SO4 and MgO–H2SO4 it was − 9.0 < HO ≤ − 7.9 and − 7.9 < HO ≤ − 6.9.
Discussion
One of the methods of increasing the acidity of metal oxides is the deposition of groups characteristic of strong mineral acids on their surface. Such surface species include, for example, the –SO3H group. This is usually done by reacting an oxide support with chlorosulfonic acid (Eq. 1) which is usually dissolved in a solvent. As a result of the reaction in which the surface hydroxyl group is involved, hydrogen chloride and the moiety (I) with the following structure are formed:
Here M is a metal from a support and Oδ− is a lattice oxide anion.
These centers are characterized by high acid strength, depending on the basicity of the oxide and hence on the value of the negative charge (δ−) located on the oxygen forming the anionic sub-lattice. The oxides of low acid strength and widely differing acid–base properties such as SiO2, TiO2, Al2O3 and MgO were chosen as supports. Their basic strength was approximated by the partial negative charge located on oxygen, which was calculated using the Sanderson method [48] and also determined by the adsorption of indicators with a known pKa value [46]. On the other hand, the acid strength was determined experimentally using the test reaction method [40] (Table 1). The obtained results indicate that selected oxides i.e. SiO2, Al2O3 and MgO possess low acid strength of HO > − 3.3, while in the case of TiO2 the value was higher at − 5.3 < HO ≤ − 3.3. The basic strength of the supports as determined by adsorption of indicators for SiO2, Al2O3, TiO2 was 7.2 ≤ H_ < 9.3 and for MgO it was 26.5 ≤ H_ < 33.0. Since indicator adsorption may give uncertain results [40] the calculation of Sanderson partial negative charges located on oxygen were performed. The results more precisely evaluated the basic properties of oxides than the indicator adsorption method (Table 1):
The results of the acid strength measurement, of systems obtained by reacting chlorosulfonic acid with inorganic carriers show clearly the effect of the basicity of oxide anions on the acid strength of the surface hydrogen sulfate group (I). The less basic oxygen of SiO2 (δ− = − 0.23) interacts weakly with the proton of the acid center (I) leading to a high acid strength of − 11.5 < HO ≤ − 10.8. The more basic oxygens of TiO2 (δ− = − 0.27), Al2O3 (δ− = − 0.36) and MgO (δ− = − 0.50) bind the proton of the hydrogen sulfate group stronger, thereby lowering the acid strength to − 9.3 < HO ≤ − 9.0. This is shown in Fig. 4.
The same value of acid strength i.e. − 9.3 < HO ≤ − 9.0 was obtained when sulfuric acid was used as the sulfonating agent for SiO2 and TiO2. The water formed in reactions (3) and (4) between SiO2 and H2SO4 could interact with the protons of the hydrogen sulfate groups thereby lowering their acid strength from the − 11.5 < HO ≤ − 10.8 level for SiO2-ClSO3H system to the − 9.3 < HO ≤ − 9.0 range. A similar phenomenon can be observed by comparing the acid strength of the sulfonic groups in Amberlyst 15 resin and in p-xylene-2-sulfonic acid dihydrate. In the first case, the strength was − 9.3 < HO ≤ − 9.0 while in the second it diminished to − 7.9 < HO ≤ − 6.9. The oxygen atoms of the most basic supports i.e. Al2O3 and MgO caused a decrease of the acid strength of the carrier-H2SO4 system to the range of − 9.0 < HO ≤ − 7.9 and − 7.9 < HO ≤ − 6.9. The results indicate that the acid strength of silica treated with chlorosulfonic acid (so called solid sulfuric acid—SSA) is higher than that of HZSM-5 and amorphous silicaalumina. The use of other oxides such as TiO2, Al2O3 and MgO led to catalysts of lower acid strength, comparable to Amberlyst 15 and HZSM-5 but higher than p-xylene-2-sulfonic acid.
Conclusions
The measurements of the minimum acid strength (HOmin) required in test reactions for initiation of cis-stilbene at 303 K and tert-butylbenzene at 313 K, 323 K and 333 K have made it possible to establish a much more precise acid strength scale. The obtained HOmin values were: − 9.0 (cis-stilbene, 303 K), − 9.3, − 9.5 and − 9.9 (tert-butylbenzene, 313 K, 323 K, and 333 K). The updated test reaction method was used to determine the acid strength of different catalysts obtained in the reaction of chlorosulfonic and sulfuric acid on different oxide supports. It was found that the acid strength values depend on the basicity of the studied supports. For the supports treated with chlorosulfonic acid, the values of acid strength were: − 11.5 < HO ≤ − 10.8 for the less basic SiO2, − 9.3 < HO ≤ − 9.0 for the more basic TiO2, Al2O3 and MgO. For the supports treated with sulfuric acid, the acid strength was: − 9.3 < HO ≤ − 9.0 for the less basic SiO2 and TiO2, − 9.0 < HO ≤ − 7.9, − 7.9 < HO ≤ − 6.9 for more basic Al2O3 and − 7.9 < HO ≤ − 6.9 for the most basic MgO. Chlorosulfonic acid treated silica possesses higher acid strength than HZSM-5 and amorphous silicaalumina. The modification of TiO2, Al2O3 and MgO with chlorosulfonic acid led to catalysts of lower acid strength, comparable to Amberlyst 15 and HZSM-5 − 9.3 < HO ≤ − 9.0, but higher than p-xylene-2-sulfonic acid dihydrate (− 7.9 < HO ≤ − 6.9).
References
Weissermel K, Arpe H-J (1993) Industrial organic chemistry. VCH, Weinheim
Surya Olah GA, Prakash GK, Molnar A, Sommer J (2009) Superacid chemistry. Wiley, New Jersey
Varadaray R, Bock J, Valint P Jr, Zushma S, Thomas R (1991) Fundamental interfacial properties of alkyl-branched sulfate and ethoxy sulfate surfactants derived from Guerbet alcohols. 1. Surface and instantaneous interfacial tensions. J Phys Chem 95:1671–1676
Egan RR, Earl GW, Ackerman J (1984) Properties and uses of some unsaturated fatty alcohols and their derivatives. J Am Oil Chem Soc 61:324–329
Feigenbaum J, Neuberg CA (1941) Simplified method for the preparation of aromatic sulfuric acid esters. J Am Chem Soc 63:3529–3530
Zolfigol MA, Khazaei A, Mokhlesi M, Derakhashan-Panah F (2013) Synthesis, characterization and catalytic properties of monodispersed nano-sphere silica sulfuric acid. J Mol Catal A 370:111–116
Sharghi H, Savari MH, Eskandari R (2005) Alumina sulfuric acid as a novel heterogeneous system for esterification of carboxylic acids in solvent free condition. J Chem Res 8:488–491
Amoozadeh A, Azadeh RA, Rahmani S, Salehi M, Kubicki M, Dutkiewicz G (2015) Nano-titania-supported sulfonic-acid-catalyzed synthesis of 2-arylbenzothiazole derivatives under solvent free conditions. Phosphorous Sulfur Silicon Related Elements 190:1874–1883
Amoozadeh A, Tabrizian E, Rahmani S (2015) Nano titania-supported sulfonic acid catalyzed synthesis of α, α′-bis(substituted-benzylidene)cycloalkanones and of their xanthene derivatives under solvent-free conditions. C R Chim 18:848–857
Shirini F, Abedini M, Kiaroudi SA (2014) Introduction of titania sulfonic acid (TiO2–SO3H) as a new, efficient, and reusable heterogeneous solid acid catalyst for the synthesis of biscoumarins. Phosphorous Sulfur Silicon Related Elem 189:1279–1288
Azarifar D, Khatami SM, Zolfigol MA, Neat-Yami R (2014) Nano-titania sulfuric acid-promoted synthesis of tetrahydrobenzo[b]pyran and 1,4-dihydropyrano[2,3-c]pyrazole derivatives under ultrasound irradiation. J Iran Chem Soc 11:1223–1230
Massah AR, Kalbasi RJ, Shafiei A (2012) ZSM-5-SO3H as a novel, efficient, and reusable catalyst for the chemoselective synthesis and deprotection of 1,1-diacetates under eco-friendly conditions. Monatsh Chem 143:643–652
Kalbasi RJ, Massah AR, Shafiei A (2011) Synthesis and characterization of BEA-SO3H as an efficient and chemoselective acid catalyst. J Mol Catal A 335:51–59
Shirini F, Mamaghani M, Atghia SV (2011) Sulfonic acid-functionalized ordered nanoporous Na+-montmorillonite (SANM): a novel, efficient and recyclable catalyst for the chemoselective N-Boc protection of amines in solventless media. Catal Commun 12:1088–1094
Shirini F, Mamaghani FM, Seddighi M (2013) Sulfonated rice husk ash (RHA-SO3H): a highly powerful and efficient solid acid catalyst for the chemoselective preparation and deprotection of 1,1-diacetates. Catal Commun 36:31–37
Yadav GD, Sharma RV (2014) Synthesis, characterization and applications of highly active and robust sulfated Fe–TiO2 catalyst (ICT-3) with superior redox and acidic properties. J Catal 311:121–128
Harmer MA, Sun Q (2001) Solid acid catalysis using ion-exchange resins. Appl Catal A 221:45–62
Rahmani S, Amoozadeh A, Kolvari E (2014) Nano titania-supported sulfonic acid: an efficient and reusable catalyst for a range of organic reactions under solvent free conditions. Catal Commun 56:184–188
Marcilly C (2006) Acido-basic catalysis. Editions Technip, Paris
Haase F, Sauer J (1998) The surface structure of sulfated zirconia: periodic ab initio study of sulfuric acid adsorbed on ZrO2(101) and ZrO2(001). J Am Chem Soc 120:13503–13512
Breitkopf C, Papp H, Li X, Olindo R, Lercher JA, Lloyd R, Wrabetz S, Jentoft FC, Meinel K, Förster S, Schindler KM, Neddermeyer H, Widdra W, Hofmann A, Sauer J (2007) Activation and isomerization of n-butane on sulfated zirconia model systems—an integrated study across the materials and pressure gaps. Phys Chem Chem Phys 9:3600–3618
Evans PN, Albertson JM (1917) The ethyl-sulfuric acid reaction. J Am Chem Soc 39:456–461
Morrow BA, McFarlane RA, Lion M (1987) An infrared study of sulfated silica. J Catal 107:239–323
Xu H, Guo D, Jiang Q, Ma Z, Li W, Wang Z (2006) Catalytic performance of sulfated silica MCM-41 for the cyclization of pseudoionone to ionones. Chin J Catal 27:1080–1086
Gonçalves VLG, Rodrigues RC, Lorençato R, Mota CJA (2007) Assessing the acid strength of solid acid catalysts with the use of linear free energy relationship: H/D exchange with substituted benzene derivatives. J Catal 248:158–164
Harmer MA, Sun Q, Michalczyk MJ, Yang Z (1997) Unique silane modified perfluorosulfonic acids as versatile reagents for new solid acid catalysts. Chem Commun 18:1803–1804
Melero JA, Stucky GD, van Grieken R, Morales G (2002) Direct syntheses of ordered SBA-15 mesoporous materials containing arenesulfonic acid groups. J Mater Chem 12:1664–1670
Siril PF, Davison AD, Randhawa JK, Brown DR (2007) Acid strengths and catalytic activities of sulfonic acid on polymeric and silica supports. J Mol Cat A 267:72–78
Yang P, Yu J, Wang Z, Liu Q, Yang X, Wu T (2005) Influence of preparation method on the structure and catalytic activity of supported solid sulfuric acid. React Kinet Catal Lett 85:153–159
Vedrine JC (2015) Acid-base characterization of heterogeneous catalysts: an up-to-date overview. Res Chem Intermed 41:9387–9423
Farcasiu D (2001) Reaction mechanisms on liquid and solid acids catalysts. Correlation with acidity. Catal Lett 71:95–103
Umansky B, Engelhardt J, Hall WK (1991) On the strength of solid acids. J Catal 127:128–140
Ikemoto M, Tsutsumi K, Takahashi H (1972) Acidity and acid strength of zeolite catalysts. Bull Chem Soc Jpn 45:1330–1334
Haw JF, Nicolas JB, Xu T, Beck LB, Ferguson DB (1996) Physical organic chemistry of solid acids: lessons from in situ NMR and theoretical chemistry. Acc Chem Res 29:259–267
Farcasiu D, Ghenciu A, Marino G, Rose KD (1997) Strength of solid acids and acids in solution. Enhancement of acidity of centers on solid surfaces by anion stabilizing solvent and its consequence for catalysis. J Am Chem Soc 119:11826–11831
Xu T, Munson EJ, Haw JF (1994) Toward a systematic chemistry of organic reactions in zeolites: in situ NMR studies of ketones. J Am Chem Soc 116:1962–1972
Cairon O, Thomas K, Chambellan A, Chevreau T (2003) Acid catalyzed benzene hydroconversion using various zeolites: Brønsted acidity, hydrogenation and side reactions. Appl Catal A 238:167–183 reference Lavalley JC, Jolly-Feaugas S, Janin A, Saussey J (1997) Microchim Acta 1451
Fraenkel D, Jentzsch NR, Starr CA, Nikrad PV (2010) Acid strength of solids probed by catalytic isobutane conversion. J Catal 274:29–51
Katada N, Endo J, Notsu K, Yasunobu N, Naito N, Niwa M (2004) Superacidity and catalytic activity of sulfated zirconia. J Phys Chem B 104:10321–10328
Marczewski M, Marczewska M, Popielarska D, Ciecierska D, Herman M, Kamińska A, Kamińska E, Wiedro R, Roguska A (2015) Styrene and styrene dimer derivatives, cyclohexene, tert-butylbenzene and cumene as test reactions for acid strength measurements of crystalline and amorphous silica-aluminas, sulfated oxides and Amberlyst. React Kinet Mech Catal 114:513–533
Marczewski M, Kavalchuk Y, Ulkowska U, Gliński M, Osawaru O (2018) Diacetone alkohol decomposition and benzaldehyde Canizzaro reaction as test reactions for basic strength measurements of alumina, magnesia, Amberlyst type resins (A-15, XN 1010, A-26, A-21), Nafion NR 50 and solid sulfuric acid. React Kinet Mech Catal. https://doi.org/10.1007/s11144-018-1492-z
Noyce DS, Hartter DR, Miles FB (1968) The kinetics and mechanism of the acid-catalyzed isomerization of cis-stilbene. J Am Chem Soc 90:4633–4637
Li XM, Huang KS, Lin M, Zhou LX (2003) Studies on formic acid-catalyzed dimerization of isorhapontigenin and of resveratrol to tetralins. Tetrahedron 59:4405–4413
Johnson DC, Katritzky AR, Shapiro SA (1969) Temperature variation of the H0 acidity function in aqueous sulfuric acid solution. J Am Chem Soc 91:6654–6662
Arnett EM, Bushick RD (1964) Solvent effects in organic chemistry. III. Solvation of stable carbonium and ammonium ions in water. The temperature coefficient of the HR acidity scale. J Am Chem Soc 86:1564–1571
Olah GA, Molnàr A (1995) Hydrocarbon chemistry. Wiley, New York, p 174
Tanabe K, Misono M, Hattori H (1989) New solid acids and bases and their catalytic properties. Elsevier, New York
Sanderson RT (1960) Chemical periodicity. Reinhold Publishing Company, New York
Acknowledgements
This work was sponsored by Faculty of Chemistry, Warsaw University of Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
OpenAccess This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Marczewski, M., Aleksandrowicz, M., Brzezińska, E. et al. Acid strength measurements of Amberlyst 15 resin, p-xylene-2-sulfonic acid and chlorosulfonic and sulfuric acid treated SiO2, Al2O3, TiO2 and MgO. Reac Kinet Mech Cat 126, 1081–1096 (2019). https://doi.org/10.1007/s11144-019-01551-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-019-01551-7