Abstract
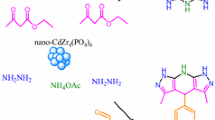
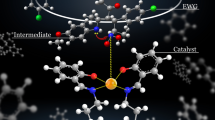
Ca9.5Mg0.5(PO4)5.5(SiO4)0.5F1.5 was used as a nano cooperative catalyst for the synthesis of dihydropyrano[2,3-c]pyrazoles derivatives via a one-pot four component reaction of aldehyde, ethyl acetoacetate, malononitrile and hydrazine hydrate. The reactions were carried out at 70 °C under mild and heterogeneous conditions. The catalyst was easily synthesized by high energy ball milling and the effect of some parameters such as specific surface area, pore volume, particle size, pH and crystallinity degree of the catalyst was studied on the yield and reaction time. The used catalyst showed both Brønsted base and Lewis acid properties. The acidic sites were determined by FT-IR spectroscopy of pyridine adsorption technique at different temperature. Using a new cooperative catalyst and an environmentally benign procedure, good to high yield of the products and relatively short reaction time were the advantages of the present method.









Similar content being viewed by others
References
Li M, Fang Z, Smith RL, Yang S (2016) Prog Energy Combust Sci 55:98–194
Peters R (2015) Cooperative catalysis. Wiley, Weinheim
Descamps M, Hornez JC, Leriche A (2009) J Eur Ceram Soc 29:369–375
Mazaheri M, Haghighatzadeh M, Zahedi AM, Sadrnezhaad SK (2009) J Alloys Compd 471:180–184
Arami H, Mohajerani M, Mazloumi M, Khalifehzadeh R, Lak A, Sadrnezhaad SK (2009) J Alloys Compd 469:391–394
Wang J, Chao Y, Wan Q, Zhu Z, Yu H (2009) Acta Biomater 5:1798–1807
Mostafa NY, Hassan HM, Mohamed FH (2009) J Alloys Compd 479:692–698
Chen Y, Miao X (2004) Ceram Int 30:1961–1965
Basar B, Tezcaner A, Keskin D, Evis Z (2010) Ceram Int 36:1633–1643
Turkoza M, Atillab AO, Evisc Z (2013) Ceram Int 39:8925–8931
Chen Y, Miao X (2005) Biomaterials 26:1205–1210
Eslami H, Solati-Hashjin M, Tahriri M (2009) Mater Sci Eng C 29:1387–1398
Fathi MH, Mohammadi Zahrani E (2009) J Cryst Growth 311:1392–1403
Suchanek WL, Byrappa K, Shuk P, Riman RE, Janas VF, TenHuisen KS (2004) Biomaterials 25:4647–4657
Mroz W, Bombalska A, Burdynska S, Jedynski M, Prokopiuk A, Budner B, Slosarczyk A, Zima A, Menaszek E, Scisłowska-Czarnecka A, Niedzielski K (2010) J Mol Struct 977:145–152
Webster TJ, Ergun C, Doremus RH, Bizios R (2002) J Biomed Mater Res 59:312–317
Sun ZP, Ercan B, Evis Z, Webster TJ (2010) J Biomed Mater Res 94A:806–815
Kheradmandfard M, Fathi MH (2010) J Alloys Compd 504:141–145
Cai Y, Zhang S, Zeng X, Wang Y, Qian M, Weng W (2009) Thin Solid Films 517:5347–5351
Portera AE, Patela N, Skepper JN, Best SM, Bonfield W (2003) Biomaterials 24:4609–4620
Balamurugan A, Rebelo AHS, Lemos AF, Rocha JHG, Ventura JMG, Ferreira JMF (2008) Dent Mater 24:1374–1380
Hijon N, Cabanas MV, Pena J, Vallet-Regi M (2006) Acta Biomater 2:567–574
Aminian A, Solati-Hashjin M, Samadikuchaksaraei A, Bakhshi F, Gorjipour F, Farzadi A, Moztarzadeh F, Schmucker M (2011) Ceram Int 37:1219–1229
Ahmadia T, Monshi A, Mortazavi V, Fathi MH, Sharifi S, HashemiBeni B, MoghareAbed A, Kheradmandfard M, Sharifnabi A (2014) Ceram Int 40:8341–8349
Ragel CV, Vallet-Regí M, Rodríguez-Lorenzo LM (2002) Biomaterials 23:1865–1872
Fathi MH, Zahrani EM (2008) Iran J Pharm Res 4:209–213
El-Tamany ES, El-Shahed FA, Mohamed BH (1999) J Serb Chem Soc 64:9–18
Ismail ZH, Aly GM, El-Degwi MS, Heiba HI, Ghorab MM (2003) Egypt J Biotechnol 13:73–82
Abdelrazak FM, Metz P, Kataeva O, Jager A, El-Mahrouky SF (2007) Arch Pharm Chem Life Sci 340:543–548
Kuo SC, Huang LJ, Nakamura H (1984) J Med Chem 27:539–544
Zaki MEA, Soliman HA, Hiekal OA, Rashad AEZ (2006) Naturoforsch C61:1–6
Wang JL, Liu D, Zheng ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z (2007) Proc Natl Acad Sci USA 97:7124–7129
Foloppe N, Fisher LM, Howes R, Potter A, Robertos AGS, Surgenor AE (2006) Bioorg Med Chem 14:4792–4802
Kashtoh H, Muhammad MT, Khan JJA, Rasheed S, Khan A, Perveen S, Javaid K, Wahab AT, Khan KM, Choudhary MI (2016) Bioorg Chem 65:61–72
Litvinov YM, Shestopalov AA, Rodinovskaya LA, Shestopalov AM (2009) J Comb Chem 11:914–919
Litvinov YM, Rodinovskaya LA, Shestopalov AM (2009) Russ Chem Bull Int Ed 58:2362–2368
Vasuki G, Kumaravel K (2008) Tetrahedron Lett 49:5636–5638
Mecadon H, Rohman MR, Kharbangar I, Laloo BM, Kharkongor I, Rajbangshi M, Myrboh B (2011) Tetrahedron Lett 52:3228–3231
Siddekha A, Nizam A, Pasha MA (2011) Spectrochim Acta A 81:431–440
Kanagaraj K, Pitchumani K (2010) Tetrahedron Lett 51:3312–3316
Mecadon H, Rohman MR, Rajbangshi M, Myrboh B (2011) Tetrahedron Lett 52:2523–2525
Kumar GS, Kurumurthy C, Veeraswamy B, Rao PS, Narsaiah B (2013) Organic Prep Proc Int 45:429–436
Chavan HV, Babar SB, Hoval RU, Bandgar BP (2011) Bull Korean Chem Soc 32:3963–3966
Bihani M, Bora PP, Bez G, Askari H (2013) Sustain Chem Eng 1:440–447
Khurana JM, Chaudhary A (2012) Green Chem Lett Rev 5:633–638
Ebrahimi J, Mohanndi A, Pakjoo V, Bahramzadeh E, Habibi A (2012) J Chem Sci 124:1013–1017
Khazdooz L, Zarei A (2016) Iran J Catal 6:69–74
Guo RY, An ZM, Mo LP, Yang ST, Liu HX, Wang SX, Zhang ZH (2013) Tetrahedron 69:9931–9938
Tamaddon F, Alizadeh M (2014) Tetrahedron Lett 55:3588–3591
Maleki B, Nasiri N, Tayebee R, Khojastehnezhad A, Akhlaghi HA (2016) RSC Adv 6:79128–79134
Bora PP, Bihani M, Bez G (2013) J Mol Catal B 92:24–33
Tyagi B, Chudasama CD, Jasra RV (2006) Appl Clay Sci 31:16–28
Ho TL (1977) Hard and soft acid and bases principle in organic chemistry. Academic Press, New York
Carey FA, Sundberg RJ (1990) Advanced organic chemistry, part B: reactions and synthesis, 3rd edn. Plenum Press, New York and London
Acknowledgements
We gratefully acknowledge the funding support received for this project from the Islamic Azad University, Khorasgan and fasa Branches.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khazdooz, L., Zarei, A., Ahmadi, T. et al. Synthesis of dihydropyrano[2,3-c]pyrazoles using Ca9.5Mg0.5(PO4)5.5(SiO4)0.5F1.5 as a new nano cooperative catalyst. Reac Kinet Mech Cat 122, 229–245 (2017). https://doi.org/10.1007/s11144-017-1217-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-017-1217-8