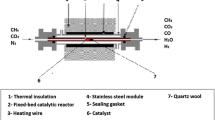
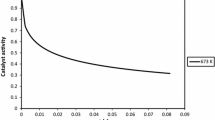
Abstract
The effect of the reaction parameters on the catalytic activity and the carbon deposition over 33 % Ni/Al2O3 catalyst was investigated. The kinetics of the CO2 reforming of methane was considered in the temperature range 450–650°C at atmospheric pressure with a 1:1:8 mixture of CH4, CO2 and N2. The reactor model for the dry reforming of methane used the Richardson and Paripatyadar kinetics and the Snoeck et al. kinetics for the coke deposition and the gasification reactions. The results led to the conclusion of the influence of CH4/CO2 ratio and temperature on the conversion/yield.










Similar content being viewed by others
Abbreviations
- DRM:
-
Dry reforming of methane
- L:
-
Reactor length (m)
- Dt :
-
Reactor external diameter (m)
- F:
-
Molar flow rate (mol s−1)
- \({\text{F}}_{{ ( {\text{j)}}}}^{\text{p}}\) :
-
Molar flow rate of production of species i (mol s−1)
- X:
-
Conversion (–)
- P:
-
Pressure (atm)
- T:
-
Temperature (K)
- Tw :
-
Wall temperature (K)
- r:
-
Specific rate of reaction (mol kg−1 s−1)
- R:
-
Universal gas constant (J mol−1 K−1)
- u:
-
Gas velocity (m s−1)
- w:
-
Transversal reactor cross section (m2)
- z:
-
Dimensionless length (–)
- ∆H:
-
Heat of reaction (kJ mol−1)
- Cp:
-
Heat capacity (J kg−1 K−1)
- Uw :
-
Global heat transfer coefficient (W m−2 K−1)
- ρg :
-
Volumetric mass density of gas (kg m−3)
- ρb :
-
Volumetric mass density of catalyst (kg m−3)
- k:
-
Reaction rate constant
- K:
-
Adsorption constant
- Kp :
-
Equilibrium constant for reaction
References
Sierra Gallego G, Batiot-Dupeyrat C, Barrault J, Mondragon F (2008) Dual active site mechanism for dry methane reforming over Ni/La2O3 produced from LaNiO3 perovskite. Ind Eng Chem Res 47:9272–9278
Quiroga MM, Castro Luna AE (2007) Kinetic analysis of rate data for dry reforming of methane. Ind Eng Chem Res 46:5265–5270
Mark MF, Mark F, Maier WF (1997) Reactions kinetics of the CO2 reforming of methane. Chem Eng Technol 20:109–116
Menegazzo F, Signoretto M, Pinna F, Cauton P, Pernicone N (2012) Optimization of bimetallic dry reforming catalysts by temperature programmed reaction. Appl Catal A 439–440:80–87
De Liobet S, Pinilla JL, Moliner R, Suelves I (2015) Relationship between carbon morphology and catalyst deactivation in the catalytic decomposition of biogas using Ni, Co and Fe based catalysts. Fuel 139:71–78
Budiman AW, Song SH, Chang TS, Shin CH, Choi MJ (2012) Dry reforming of methane over cobalt catalysts a literature review of catalyst development. Catal Surv Asia 16:183–197
Guo J, Lou H, Zhao H, Chai D, Zheng X (2004) Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl Catal A 273:75–82
Corthals S, Van Nederkassel J, Geboers J, De Winne H, Van Noyen J, Moens B, Sels B, Jacobs P (2008) Influence of composition of MgAl2O4 supported NiCeO2ZrO2 catalysts on coke formation and catalyst stability for dry reforming of methane. Catal Today 138:28–32
Tsang SC, Claridge JB, Green MLH (1995) Recent advances in the conversion of methane to synthesis gas. Catal Today 23:3–15
Gronchi P, Mazzocchia M, Del Rosso R (1995) Carbon dioxide reaction with methane on La2O3 supported Rh catalysts. Energy Convers Manage 36:605–608
Bychkov VY, Tyulenin YP, Firsova AA, Shafranovsky EA, Gorenberg A Ya, Korchak VN (2013) Carbonization of nickel catalysts and its effect on methane dry reforming. Appl Catal A 453:71–79
Nagaoka K, Seshan K, Lercher JA, Aika K (2000) Activation mechanism of methane derived coke (CHx) by CO2 during dry reforming of methane comparison for Pt/Al2O3 and Pt/ZrO2. Catal Lett 70:109–116
Chen D, Lodeng R, Anundskas A, Olsvik O, Holmen A (2001) Deactivation during carbon dioxide reforming of methane over Ni catalyst: microkinetic analysis. Chem Eng Sci 56:1371–1379
Park N, Park M, Back S, Sutta K, Lee Y-J, Kwak G, Park H, Jun K (2014) Modeling and optimization of the mixed reforming of methane: maximizing CO2 utilization for non-equilibrated reaction. Fuel 115:357–365
Richardson JT, Paripatyadar SA (1990) Carbon dioxide reforming of methane with supported rhodium. Appl Catal 61:293–309
Wang S, Lu GQ (1996) Carbon dioxide reforming of methane to produce synthesis gas over metal-supported catalysts: state of the art. Energy Fuels 10:896–904
Agrawal V, Chaudra V (2014) Simulation of fluidized bed reactor for producing synthesis gas by catalytic CH4–CO2 reforming. J CO2 Util 5:10–16
Istadi I, Anggoro DD, Amin NAS, Ling DHW (2011) Catalyst deactivation simulation through carbon deposition in carbon dioxide reforming over Ni/CaO–Al2O3 catalyst. Bull Chem React Eng Catal 6:129–136
Rostrup-Nielson JR (1983) Catalytic steam reforming, vol 5. Catalysis Science and Technology, Springer, pp 1–118
Snoeck J-W, Froment GF, Fowles M (1997) Filamentous carbon formation and gasification: thermodynamics, driving force, nucleation and steady-state growth. J Catal 169:240–249
Snoeck J-W, Froment GF, Fowles M (2002) Steam/CO2 reforming of methane. Carbon filament formation by the Boudouard reaction and gasification by CO2, by H2, and by steam: kinetic study. Ind Eng Chem Res 41:4252–4265
Benguerba Y, Dehimi L, Virginie M, Dumas C, Ernst B (2015) Modelling of methane dry reforming over Ni/Al2O3 catalyst in a fixed-bed catalytic reactor. Reac Kinet Mech Cat 114:109–119
Serrano-Lotina A, Daza L (1995) Influence of the operating parameters over dry reforming of methane to syngas. Int J Hydrogen Energ 39:605–608
Al-Ali K, Kodama S, Sekiguchi H (2014) Modeling and simulation of methane dry reforming in direct contact bubble reactor. Sol Energy 102:45–55
Fakeeha AH, Alfatish AS, Soliman MA, Ibrahim AA (2006) Effect of changing CH4/CO2 ratio on hydrogen production by dry reforming reaction. 16th WHEC, Lyon, France, 13–16 June 2006
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Benguerba, Y., Dehimi, L., Virginie, M. et al. Numerical investigation of the optimal operative conditions for the dry reforming reaction in a fixed-bed reactor: role of the carbon deposition and gasification reactions. Reac Kinet Mech Cat 115, 483–497 (2015). https://doi.org/10.1007/s11144-015-0849-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-015-0849-9