Abstract
Purpose
Exacerbations of chronic obstructive pulmonary disease (COPD) are associated with deteriorating health and health-related quality of life (HRQoL) among people with COPD during and after events. HRQoL data are key to evaluating treatment cost-effectiveness and informing reimbursement decisions in COPD. EuroQoL 5-dimension 5-level (EQ-5D-5L) utility scores, based on various HRQoL measures, are used in economic evaluations of pharmacotherapy. These analyses estimated associations between EQ-5D-5L utility scores and exacerbations (new and previous) in patients with moderate-to-very severe COPD.
Methods
Longitudinal mixed models for repeated measures (MMRM), adjusted for time and treatment, were conducted using data from the ETHOS study (NCT02465567); models regressed EQ-5D-5L on current and past exacerbations that occurred during the study, adjusting for other patient reported outcomes and clinical factors.
Results
Based on the simplest covariate adjusted model (adjusted for current exacerbations and number of previous exacerbations during the study), a current moderate exacerbation was associated with an EQ-5D-5L disutility of 0.055 (95% confidence interval: 0.048, 0.062) with an additional disutility of 0.035 (0.014, 0.055) if the exacerbation was severe. After resolving, each prior exacerbation was associated with a disutility that persisted for the remainder of the study (moderate exacerbation, 0.014 [0.011, 0.016]; further disutility for severe exacerbation, 0.011 [0.003, 0.018]).
Conclusion
An EQ-5D-5L disutility of 0.090 was associated with a current severe exacerbation in ETHOS. Our findings suggest incorporating the effects of current, recently resolved, and cumulative exacerbations into economic models when estimating benefits and costs of COPD pharmacotherapy, as exacerbations have both acute and persistent effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Plain English Summary
People living with chronic obstructive pulmonary disease (COPD) experience symptoms such as coughing, difficulty breathing, and heavy mucus production. When COPD symptoms acutely worsen, the patient is said to have an “exacerbation”. Exacerbations can be “moderate”, resulting in the need for additional medicine, or “severe”, resulting in hospital admission or possibly death.
Exacerbations are often associated with lower quality of life. This study looked at how a patient’s quality of life is associated with the number and type of exacerbations they experience. Data from patients with moderate-to-very severe COPD who participated in a large clinical trial called ETHOS were used. The quality of a patient’s life was estimated using the EuroQoL 5-dimension 5-level survey, also called EQ-5D-5L. The EQ-5D-5L survey asks about aspects of daily living, such as ability to move, self-care, usual activities, pain/discomfort, and anxiety/depression. Survey answers are combined into a single score.
Findings from the study show how much lower quality of life is for patients living with COPD during and after an exacerbation. Lower quality of life is linked to the number and type of exacerbations patients experience. Measures of breathing difficulty do not necessarily fully explain the lower quality of life during and after exacerbations, indicating other factors may affect the quality of life of patients experiencing exacerbations. These findings may help healthcare providers further understand how much moderate or severe COPD exacerbations are related to a patient’s quality of life both during and after an exacerbation so appropriate courses of action can be taken.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation and respiratory symptoms, including dyspnea, cough, and sputum production [1]. Despite being preventable and treatable [1], COPD is the third leading cause of death worldwide, accounting for approximately 6% of deaths in 2019 [2]. An acute worsening of COPD symptoms (i.e., an exacerbation [1]) may be defined as moderate when managed through primary care or outpatient services (oral corticosteroids and antibiotics) or severe if hospitalization and monitoring is required [3]. COPD exacerbations negatively impact lung function [4] and are detrimental to health-related quality of life (HRQoL) [5, 6], with the effects taking weeks to months to resolve [4].
One validated tool to assess HRQoL in patients with COPD is the EuroQoL 5-dimension 5-level (EQ-5D-5L) questionnaire [7, 8]. The EQ-5D-5L examines five dimensions of life (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) across five severity levels (no problems to extreme problems) [7]. EQ-5D-5L utility scores, anchored on 0 (death) and 1 (full health), can be used in economic evaluations [7]. EQ-5D-5L utility scores are based on HRQoL measures and exacerbations negatively affect HRQoL [4]. Previous studies have reported lower EQ-5D-3L utility scores in patients experiencing moderate or severe exacerbations [9,10,11] as well as associations between EQ-5D scores and the number of previous exacerbations [12, 13]. However, information on associations between exacerbations and EQ-5D-5L utility scores is still limited.
Mapping algorithms can predict EQ-5D utility scores in patients with COPD from HRQoL measurements obtained from the St. George’s Respiratory Questionnaire (SGRQ) [14]. Additionally, a small study demonstrated COPD severity and exacerbation frequency are associated with some patient-reported HRQoL measures based on visual analog scale (VAS) and time trade-off (TTO) assessments, with estimated reductions from 1 non-serious or serious exacerbation, respectively, of 0.037 and 0.090 for the VAS and 0.010 and 0.042 for TTO [6]. Moreover, a multivariate analysis of data from a UK COPD cohort study reported EQ-5D-5L scores were lowered by 0.082 and 0.143, respectively, in patients with any exacerbation or a severe exacerbation in the past 12 months [13]. However, further research is needed to expand our understanding of the magnitude of the EQ-5D-5L disutility associated with COPD exacerbations.
In the ETHOS study, patients with moderate-to-very severe COPD randomized to twice-daily triple inhaled corticosteroid/long-acting muscarinic antagonist/long-acting β2-agonist (ICS/LAMA/LABA) therapy with budesonide/glycopyrronium/formoterol fumarate dihydrate at two doses (320/14.4/10 μg [BGF 320] or 160/14.4/10 μg [BGF 160]) experienced lower annual moderate/severe exacerbation rates than patients randomized to dual therapy with the LAMA/LABA glycopyrronium/formoterol fumarate dihydrate 14.4/10 μg (GFF) or the ICS/LABA budesonide/formoterol fumarate dihydrate 320/10 μg (BFF) [15]. Additionally, improvements in HRQoL (measured by the SGRQ) favored BGF 320 and BGF 160 over GFF and BFF after 24 or 52 weeks of treatment [16].
The current analyses aimed to examine associations between COPD exacerbations and EQ-5D-5L utility scores in patients with moderate-to-very severe COPD from the ETHOS study using three questions. First, what is the magnitude of the association between exacerbations (both current and previous during the study) and EQ-5D-5L disutility? Second, to what extent do these associations exist conditionally based on lung function (measured by forced expiratory volume in 1 s [FEV1]) or HRQoL (measured by SGRQ). Finally, can EQ-5D-5L utility scores be predicted based on lung function, recent exacerbation history, and current exacerbation status? Although this work uses ETHOS data, the analyses examined general relationships between the EQ-5D-5L and exacerbations, rather than elucidating the benefits of BGF.
Methods
Population
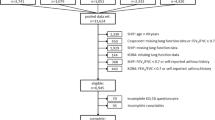
These analyses utilized patients from the randomized, double-blind, parallel-group, Phase III, 52-week ETHOS study (NCT02465567), which assessed the efficacy and safety of BGF at two doses versus GFF or BFF in patients with COPD. Detailed descriptions of ETHOS and its population have been previously published [15, 17]. Briefly, patients were 40–80 years old, with symptomatic moderate-to-very severe COPD, had a post-bronchodilator ratio of FEV1 to the forced vital capacity of < 0.7 with a post-bronchodilator FEV1 of 25–65% of the predicted normal, and a history of ≥ 1 moderate/severe exacerbations (if FEV1 was < 50% of predicted normal) or ≥ 2 moderate exacerbations/ ≥ 1 severe exacerbation (if FEV1 was > 50% of predicted normal) in the year before screening [15]. Moderate exacerbations were defined as those leading to treatment with systemic glucocorticoids, antibiotics, or both for at least three days; severe exacerbations were those resulting in hospitalization (including to an emergency room or equivalent healthcare facility) or death.
Patients were randomized 1:1:1:1 to BGF 320/14.4/10 μg, BGF 160/14.4/10 μg, GFF 14.4/10 μg, or BFF 320/10 μg twice-daily over 52 weeks via a single metered dose inhaler [15]. These analyses used the modified intention-to-treat population (mITT; all patients with post-randomization data obtained before study drug discontinuation) of 8509 patients, of which 8498 patients had EQ-5D-5L, SGRQ, or FEV1 data; data from 11 patients did not contribute to the analyses. Data from 3316 patients with ≥ 1 post-randomization FEV1 assessment were available for analyses that included FEV1 assessment across multiple timepoints, with the majority being from the ETHOS pulmonary function sub-study (n = 3088 patients) [18].
Model structure
Longitudinal models with EQ-5D-5L utility scores as the response were fitted to answer three questions. All models used mixed model for repeated measures (MMRM) with unstructured covariance matrices for EQ-5D-5L utility scores and were adjusted for time (visits; baseline and five post-baseline visits) and post-baseline treatment effects. The models included indicators for each post-baseline visit with interactions by treatment group, resulting in different average outcomes for each treatment group at each post-baseline visit. The same base model was used across questions, allowing for correlations among EQ-5D-5L utility scores within a patient [19]. Regression equations for each model are provided in Online resource 1. In Questions 1 and 2, the estimated population average effect of moderate and severe exacerbations was examined. Though adjusted for treatment, these analyses investigated associations between EQ-5D-5L and exacerbations rather than evaluating the benefits of BGF.
Question 1: What is the magnitude of the association between exacerbations and EQ-5D-5L utility scores?
Three models explored Question 1 (Table 1). Model 1 included covariates for a current exacerbation (moderate or severe) and for a current severe exacerbation. The regression coefficient for the first covariate is the difference in EQ-5D-5L utility associated with a moderate exacerbation, and the regression coefficient for the second covariate is the additional difference associated with a severe exacerbation. The sum of these two coefficients is the difference associated with a severe exacerbation. We use the term “disutility” to refer to a difference with its sign reversed. Model 1 also included a covariate for the number of previous exacerbations (moderate or severe) during the study and a covariate for the number of previous severe exacerbations during the study. Models 2 and 2a included the same exacerbation covariates as Model 1, with additional baseline covariates (see Table 1). Model 2 used FEV1 percentage of predicted normal (FEV1% predicted) as a continuous variable; Model 2a used FEV1% predicted as a categorical variable (moderate COPD [reference category], severe COPD, very severe COPD). Severity based on FEV1% predicted was based on Global Initiative for Chronic Obstructive Lung Disease (GOLD) definitions (moderate FEV1% predicted, ≥ 50% and < 80%; severe FEV1% predicted, ≥ 30% and < 50%; very severe FEV1% predicted, < 30%) [1]. These models included data from 8498 patients with EQ-5D-5L utility scores from the mITT population.
Question 2: To what extent is the association between exacerbations and EQ-5D-5L utility scores explained by the associations between exacerbations and either SGRQ or FEV1?
Four models explored Question 2 (Table 1). Model 3 included the same covariates as Model 2a, with the addition of SGRQ total score by visit and its squared value by visit as time-varying covariates, as SGRQ and its squared value are important predictors of utility [14]. Model 3 included data from the same 8498 patients used in Models 1 and 2.
Models 4 and 4a included current exacerbations (moderate or severe) and the number of previous exacerbations (moderate or severe) during the study (describing the association of EQ-5D-5L with an exacerbation), all baseline variables included in Models 2 and 2a, respectively, and current FEV1 severity as continuous (Model 4; based on post-bronchodilator FEV1% predicted) or categorical (Model 4a; moderate COPD [reference category], severe, and very severe COPD) covariates. Models 4 and 4a examined whether associations between EQ-5D-5L utility scores and exacerbations were retained after adjusting for current FEV1 severity or if they existed conditionally based on FEV1 severity. Model 4b included the same covariates as Model 4a, with the addition of SGRQ total score by visit and its squared value by visit as time-varying covariates to examine whether associations between EQ-5D-5L utility scores and exacerbations were retained after adjusting for current FEV1 severity and SGRQ total score. Data from 3316 patients with EQ-5D-5L utility scores and FEV1 information were used in Models 4, 4a, and 4b. As there were fewer severe exacerbations than moderate exacerbations in this population, Models 4, 4a, and 4b did not include covariates for current severe exacerbations or the number of previous severe exacerbations during the study. Hence, covariate effects for associations between exacerbations and EQ-5D-5L utility scores included exacerbations independent of severity.
Question 3: What would be the predicted EQ-5D-5L utility score for a given disease severity and exacerbation status (current and previous)?
Model 5 (Table 1) explored Question 3 and included visit, post-baseline treatment effects, number of previous exacerbations during the study, and the interaction between a current exacerbation (yes/no) and current FEV1 severity (categorical: moderate COPD [reference category], severe, and very severe COPD). The model allowed for different EQ-5D-5L utility scores for each current FEV1 level and for different disutilities of current exacerbations by FEV1 level. The model assumed the association between the cumulative number of previous exacerbations during the study and EQ-5D-5L utility score was the same across FEV1 severity levels, reflecting the disutility of HRQoL during exacerbations and after they have subsided, regardless of FEV1 severity level. As for Models 4, 4a, and 4b, Model 5 used data from 3316 patients with EQ-5D-5L utility scores and FEV1 information and did not include covariates for current severe exacerbations or the number of previous severe exacerbations during the study. The objective of Question 3 differed from Question 1 in that Question 3 aimed to predict individual EQ-5D-5L scores from exacerbation status and FEV1, as would be useful in a health economic model, rather than in investigating population level associations.
Assumptions and handling of missing data
The analyses assumed missing data (EQ-5D-5L utility scores and SGRQ scores) were missing at random (MAR; i.e., the probability of missing data does not depend on unobserved data). A sensitivity analysis using a data not missing at random (MNAR) assumption was completed to support Model 5 (see online resources for full details) due to concerns that EQ-5D-5L and SGRQ scores are more likely to be missing for patients with poorer HRQoL, which would result in positive bias (i.e., an upward shift) in the predicted baseline covariate identified in the model.
Results
Population characteristics
Across ETHOS treatment groups, mean age was 64.7 years, 59.7% of patients were male, and mean post-rescue medication FEV1% predicted was 43.4% in the mITT population (Online resource 2). In the 12 months preceding the study, patients in each treatment group averaged 1.7 exacerbations, with approximately 21% of patients having ≥ 1 severe exacerbation. Across treatment groups, 47.8–50.9% of patients had ≥ 1 exacerbation during the study.
Question 1: What is the magnitude of the association between exacerbations and EQ-5D-5L utility scores?
When only exacerbation covariates were considered, an EQ-5D-5L disutility of 0.055 was associated with a current moderate exacerbation during a visit compared with a visit without a current exacerbation (Fig. 1a [Model 1]). Severe exacerbations were associated with an additional EQ-5D-5L disutility of 0.035, totalling a disutility of 0.090. Each previous moderate exacerbation during the study was associated with an EQ-5D-5L disutility of 0.014, with an additional disutility of 0.011 for each previous severe exacerbation during the study. Accordingly, the total disutility of a previous severe exacerbation during the study was 0.024 (0.017, 0.032). These coefficients indicate both immediate (disutility of 0.055 for an acute moderate event) and lasting (disutility of 0.014 remaining after moderate event resolution) associations of exacerbations on EQ-5D-5L utility scores. EQ-5D-5L disutilities persisted and were similar when including baseline covariates in the models (Fig. 1b and 1c [Models 2 and 2a]).
Coefficients of exacerbation variables for EQ-5D-5L utility scores (Question 1: Models 1, 2, and 2a)a,b. Note: EQ-5D-5L disutility score for FEV1 (continuous) represents the disutility associated with a 1% reduction in FEV1 Error bars indicate the 95% CI Wald p-values; *p < 0.05, **p < 0.01 Values for the total effect of a current or previous severe exacerbation may not equal the sum of the disutilities associated with a moderate exacerbation and the additional disutility if the exacerbation is severe due to rounding. aModels include covariates as outlined in Table 1. bBased on 8498 patients CI confidence interval, EQ-5D-5L EuroQoL 5-dimension 5-level, FEV1% predicted forced expiratory volume in 1 s percentage of predicted normal value
Question 2: To what extent is the association between exacerbations and EQ-5D-5L utility scores explained by the associations between exacerbations and either SGRQ or FEV1?
When SGRQ score was included as a covariate (Model 3), in addition to the covariates included in Model 2a, disutilities (95% confidence interval [CI]) associated with SGRQ and SGRQ squared values were 0.0027 (0.0023, 0.0030) and 0.000035 (0.000031, 0.000039) per unit increase in SGRQ and SGRQ squared, respectively, indicating larger SGRQ scores are associated with lower EQ-5D-5L utility. In this model, a current moderate exacerbation was associated with a disutility (95% CI) of 0.012 (0.005, 0.019), with an additional disutility of 0.021 (0.002, 0.039) if the exacerbation was severe, totalling 0.033 (0.015, 0.050) for a current severe exacerbation. Notably, EQ-5D-5L disutilities (95% CI) associated with previous exacerbations during the study were not observed when including SGRQ in the model (disutility for each previous moderate exacerbation: 0.001 [–0.001, 0.004]; additional disutility if severe: 0.003 [–0.004, 0.009]; total disutility for severe exacerbation: 0.004 [–0.002, 0.011]). Using a similar model, where current continuous post-bronchodilator FEV1% predicted was used as a covariate rather than a baseline categorical variable, comparable results were observed but, for brevity, are not reported. Together, these results suggest SGRQ and its squared value are good predictors that may partially account for associations between EQ-5D-5L utility scores and exacerbations because the magnitude of the associations was smaller when included in the model.
When current FEV1 severity was added as a covariate, associations between EQ-5D-5L utility scores and current exacerbations and the number of previous exacerbations during the study were retained (Fig. 2), suggesting FEV1 does not fully account for associations between exacerbations and EQ-5D-5L utility scores. In the model with continuous FEV1 as a covariate (Model 4), current exacerbations were associated with an EQ-5D-5L disutility of 0.024, with an additional disutility of 0.008 for each previous exacerbation during the study (Fig. 2a). In the model with categorical FEV1 as a covariate (Model 4a), severe or very severe current FEV1 was associated with EQ-5D-5L disutilities of 0.018 or 0.045, respectively, compared with moderate current FEV1 severity. Current exacerbations were associated with an EQ-5D-5L disutility of 0.027, with an additional disutility of 0.009 for each previous exacerbation (Fig. 2b). When SGRQ was included as a covariate (Model 4b), in addition to the covariates included in Model 4a, disutilities (95% CI) of 0.0026 (0.019, 0.0033) and 0.000032 (0.000025, 0.000039) were associated with an increase in SGRQ and SGRQ squared per unit, respectively. Notably, in this model, associations between a current exacerbation (disutility [95% CI]: –0.004 [–0.015, 0.007]) and each previous exacerbation during the study (0.002 [–0.001, 0.005]) were not observed when SGRQ was included. Disutilities associated with severe and very severe FEV1, respectively, were 0.004 (–0.003, 0.011) and 0.012 (0.002, 0.022). These results indicate the association of a current exacerbation and EQ-5D-5L is not fully accounted for by the SGRQ (Model 3) or by FEV1 severity (Models 4 and 4a) alone, but may be fully accounted for when both SGRQ and FEV1 severity are included (Model 4b).
Coefficients for associations of exacerbations with EQ-5D-5L utility scores when accounting for baseline covariates with a) continuous FEV1 (Question 2: Model 4) and b) FEV1 severity (Question 2: Model 4a)a,b. Note: EQ-5D-5L disutility score for FEV1 (continuous) represents the disutility associated with a 1% reduction in FEV1 Wald p-values; **p < 0.01. aModels include covariates as described in Table 1. bBased on 3316 patients. cModerate COPD (reference category). Moderate FEV1% predicted was defined as ≥ 50% and < 80%; severe FEV1% predicted was defined as ≥ 30% and < 50%; very severe FEV1% predicted was defined as < 30% CI confidence interval, EQ-5D-5L EuroQoL 5-dimension 5-level, FEV1 forced expiratory volume in 1 s, FEV1% predicted forced expiratory volume in 1 s percentage of predicted normal value
Question 3: What is the predicted EQ-5D-5L utility score for a given disease severity and exacerbation status (current and previous)?
Across all visits and treatment groups, Model 5 predicted that the EQ-5D-5L utility score among patients with moderate COPD and no current exacerbations or previous exacerbations during the study was 0.739 (Table 2), with EQ-5D-5L disutilities of 0.016 for severe COPD and 0.048 for very severe COPD. A current exacerbation was associated with an EQ-5D-5L disutility of 0.015, with additional disutilities associated with a current exacerbation based on COPD states: 0.009 (severe COPD) or 0.023 (very severe COPD). Each previous exacerbation was associated with an EQ-5D-5L disutility of 0.009, irrespective of exacerbation severity. Results from the MNAR sensitivity analysis were similar to those from the MAR model (Online resource 3).
To demonstrate the model, consider hypothetical ‘Patient A’ from the ETHOS study, who has very severe COPD, experienced one previous exacerbation during the study, and is currently experiencing an exacerbation. The model predicts the base case (moderate COPD with no exacerbations experienced during the study) EQ-5D-5L utility score is 0.739. The predicted EQ-5D-5L disutility for ‘Patient A’ would be: 0.048 for having very severe COPD, 0.009 for having one previous exacerbation during the study, and 0.015 + 0.023 for having a current exacerbation and having very severe COPD, respectively. Therefore, ‘Patient A’ would have an EQ-5D-5L utility score of 0.644 (0.739 base case − 0.095 disutility).
Discussion
These analyses considered three questions to examine associations between COPD exacerbations and EQ-5D-5L utility scores using ETHOS study data in distinct statistical models with differing covariates. Question 1 quantified the association between exacerbations and EQ-5D-5L utility scores (Model 1) and assessed if this association was robust to the inclusion of baseline demographics, clinical characteristics, and FEV1% predicted (Model 2 and 2a). Question 2 assessed if the observed associations were explained by covariates for lung function and HRQoL (Models 3–4b). Finally, in a change of objective, Question 3 aimed to predict EQ-5D-5L utility scores given current and previous exacerbation status and disease severity (Model 5).
Exacerbations of COPD were associated with acute (based on current exacerbations) and continuing (based on previous exacerbations during the study) EQ-5D-5L disutility in patients with moderate-to-very severe COPD. Severe exacerbations and more severe COPD (as measured by FEV1% predicted) were associated with further EQ-5D-5L disutility. Measures of lung function (FEV1 severity) or patient-reported measures of breathing problems (SGRQ total scores) separately did not fully explain the EQ-5D-5L disutility associated with exacerbations, but together they may. Lastly, Model 5 predicted EQ-5D-5L utility scores in patients with COPD and EQ-5D-5L disutility during or after exacerbations based on varying FEV1 severity and exacerbation history.
Our findings quantify the extent to which EQ-5D-5L disutility is associated with current exacerbations and their severity in patients with COPD. Importantly, associations of exacerbations with EQ-5D-5L utility scores remain after resolution of an exacerbation, as indicated by further EQ-5D-5L disutility with each previous exacerbation during the study. As such, it may be insufficient to consider current exacerbations and exacerbation history in isolation when estimating EQ-5D-5L disutility because the impact of current exacerbations on HRQoL may depend on exacerbation history, though not considered in previously published cost-effectiveness models for COPD [20,21,22,23]. In Markov models, patients sojourn in disease severity states, with patients developing moderate or severe exacerbations according to risks associated with each state. Costs and quality of life scores attributed to exacerbations and severity states are compared between treatment and control arms to determine incremental costs per quality-adjusted life year. The current analyses provide important new insights by demonstrating additive effects of previous exacerbations on EQ-5D-5L disutility should be considered in future evaluations. Model 5 showed the effects of exacerbations on EQ-5D-5L disutility were greater in patients with poorer lung function (as indicated by greater FEV1 severity). These findings suggest effective maintenance pharmacotherapies could preserve EQ-5D-5L utility scores in patients with COPD. Overall, these results suggest exacerbations would be associated with HRQoL disutility in patients with moderate-to-very severe COPD, even if other assessments were used. Therefore, the current results could help to improve policy and decision making through health economics evaluations.
These results are consistent with previous reports. A previous meta-analysis reported that moderate and severe exacerbations were associated with lower EQ-5D-5L, with severe exacerbations having a greater impact than moderate exacerbations [24]. In an analysis of the overall population (n = 18,746) and the population of patients with ≥ 1 prior severe exacerbations (n = 4483), an increase in moderate and severe exacerbations of one per year was associated with EQ-5D-5L index score disutilities of 0.02 and 0.03, respectively [24]. Our results are also consistent with a previous publication by Rutten-van Molken et al. that assessed the effect of COPD health status on HRQoL [6], although there are methodological differences between these analyses that limit study comparability. Rutten-van Molken et al. assessed the effect of COPD health status (based on COPD severity, non-serious exacerbations, and serious exacerbations) on HRQoL in 239 patients with COPD, as measured by VAS and TTO (as opposed to EQ-5D-5L) [6]. Their analysis demonstrated consistently lower VAS and TTO values over a one-year period for more severe COPD (mild versus severe) and as the number of exacerbations increased. For non-serious and serious exacerbations, respectively, estimated VAS disutilities were 0.037 and 0.090 and TTO disutilities were 0.010 and 0.042. So, TTO disutilities (another preference based method) were smaller in magnitude than disutilities observed in the current study (0.055 for current non-severe exacerbations; 0.090 for current severe exacerbations). However, methodologic issues make direct comparisons between these studies difficult. Rutten-van Molken et al. examined VAS and TTO values using mild COPD as the base case and enrolled patients with and without exacerbation histories [6], whereas our analysis used moderate COPD as the base case and included only patients with an exacerbation history before study enrolment. Furthermore, a benefit of the current analysis is that the EQ-5D-5L is preference based, relying on weighted estimates for each item from a large sample and for a representative sample of the general public. The EQ-5D-5L is preferred for economic evaluations for decision making over direct methods, such as VAS [25].
The strengths of this analysis include using data from large cohorts (n = 8498 or n = 3316 depending on the model), allowing for robust findings. Furthermore, the predictive model examined the joint effects of clinical dynamics (lung function) and clinical events (exacerbations) to show strong associations of exacerbations with EQ-5D-5L disutility and presumably HRQoL.
This analysis was limited by the 52-week ETHOS study duration, as the effect of exacerbations on EQ-5D-5L utility scores beyond one year of follow-up could not be evaluated. Therefore, longer-term associations between exacerbations and EQ-5D-5L utility scores could not be extrapolated. The current analysis also did not include populations beyond the ETHOS study, for example patients with mild COPD and no recent exacerbation history. Furthermore, the analysis did not account for a boundary effect, as the modeling assumptions allowed maximum EQ-5D-5L utility score to exceed 1 (a value outside the instrument’s anchored range). Finally, though the current findings demonstrate associations between exacerbations and EQ-5D-5L disutility, these analyses do not attempt to infer causation. Further investigations may be useful to assess the impact of additional covariates, infer causation, or validate the observed associations between exacerbations and EQ-5D-5L utility scores.
Conclusions
Statistical modeling indicated that current and previous exacerbations of COPD were associated with disutility on the EQ-5D-5L, which measures various aspects of HRQoL [7], in patients with moderate-to-very severe COPD from the ETHOS study. Severe exacerbations and more severe disease were associated with further EQ-5D-5L disutility. Inclusion of lung function or patient-reported measures of breathing problems alone may not fully explain the EQ-5D-5L disutility associated with exacerbations, but together they may. Importantly, the EQ-5D-5L disutility associated with a current severe exacerbation from Models 1, 2 and 2a was larger than that observed in other economic evaluations [6]. However, these findings require confirmation after accounting for COPD severity, for example by fitting a model similar to Model 5 that distinguishes between the associations of severe and non-severe exacerbations. To adequately identify this type of model, data from a sufficient number of patients with known current COPD severity and a sufficiently high rate of severe exacerbations would be required. Overall, these results suggest health economic evaluations should consider both current and previous exacerbation status, including the severity and number of exacerbations, on EQ-5D-5L when estimating the benefits of COPD pharmacotherapy, rather than just mapping from SGRQ or modeling the progression of disease severity and lung function decline. Predictive modeling of EQ-5D-5L utility using lung function and exacerbation data may help healthcare professionals to understand the HRQoL of patients with moderate-to-very severe COPD.
Data availability
Not applicable.
References
Global Initiative for Chronic Obstructive Lung Disease. (2022). 2022 GOLD Report. Global strategy for the diagnosis, management and prevention of COPD. Retrieved February 2, 2022, from https://goldcopd.org/2022-gold-reports-2/
World Health Organization. (2020, December 9th, 2020). The top 10 causes of death. Retrieved December 8, 2021, from https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
Ko, F. W., Chan, K. P., Hui, D. S., Goddard, J. R., Shaw, J. G., Reid, D. W., & Yang, I. A. (2016). Acute exacerbation of COPD. Respirology, 21(7), 1152–1165. https://doi.org/10.1111/resp.12780
Hurst, J. R., Skolnik, N., Hansen, G. J., Anzueto, A., Donaldson, G. C., Dransfield, M. T., & Varghese, P. (2020). Understanding the impact of chronic obstructive pulmonary disease exacerbations on patient health and quality of life. European Journal of Internal Medicine, 73, 1–6. https://doi.org/10.1016/j.ejim.2019.12.014
Miravitlles, M., Peña-Longobardo, L. M., Oliva-Moreno, J., & Hidalgo-Vega, Á. (2015). Caregivers’ burden in patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease, 10, 347–356. https://doi.org/10.2147/COPD.S76091
Rutten-van Mölken, M. P. M. H., Hoogendoorn, M., & Lamers, L. M. (2009). Holistic preferences for 1-year health profiles describing fluctuations in health: The case of chronic obstructive pulmonary disease. PharmacoEconomics, 27(6), 465–477. https://doi.org/10.2165/00019053-200927060-00003
EuroQol. (2019). EQ-5D-5L user guide. Retrieved November 13, 2019, from https://euroqol.org/wp-content/uploads/2019/09/EQ-5D-5L-English-User-Guide_version-3.0-Sept-2019-secured.pdf.
Nolan, C. M., Longworth, L., Lord, J., Canavan, J. L., Jones, S. E., Kon, S. S., & Man, W. D. (2016). The EQ-5D-5L health status questionnaire in COPD: Validity, responsiveness and minimum important difference. Thorax, 71(6), 493–500. https://doi.org/10.1136/thoraxjnl-2015-207782
O’Reilly, J. F., Williams, A. E., & Rice, L. (2007). Health status impairment and costs associated with COPD exacerbation managed in hospital. International Journal of Clinical Practice, 61(7), 1112–1120. https://doi.org/10.1111/j.1742-1241.2007.01424.x
Goossens, L. M., Nivens, M. C., Sachs, P., Monz, B. U., & Rutten-van Mölken, M. P. (2011). Is the EQ-5D responsive to recovery from a moderate COPD exacerbation? Respiratory Medicine, 105(8), 1195–1202. https://doi.org/10.1016/j.rmed.2011.02.018
Menn, P., Weber, N., & Holle, R. (2010). Health-related quality of life in patients with severe COPD hospitalized for exacerbations - comparing EQ-5D, SF-12 and SGRQ. Health and Quality of Life Outcomes, 8, 39. https://doi.org/10.1186/1477-7525-8-39
Esquinas, C., Ramon, M. A., Nuñez, A., Molina, J., Quintano, J. A., Roman-Rodríguez, M., Naberan, K., Llor, C., Roncero, C., Miravitlles, M., & Barrecheguren, M. (2020). Correlation between disease severity factors and EQ-5D utilities in chronic obstructive pulmonary disease. Quality of Life Research, 29(3), 607–617. https://doi.org/10.1007/s11136-019-02340-4
Hoogendoorn, M., Jowett, S., Dickens, A. P., Jordan, R., Enocson, A., Adab, P., Versteegh, M., & Mölken, M.R.-V. (2021). Performance of the EQ-5D-5L plus respiratory bolt-on in the birmingham chronic obstructive pulmonary disease cohort study. Value in Health, 24(11), 1667–1675. https://doi.org/10.1016/j.jval.2021.05.006
Starkie, H. J., Briggs, A. H., Chambers, M. G., & Jones, P. (2011). Predicting EQ-5D values using the SGRQ. Value Health, 14(2), 354–360. https://doi.org/10.1016/j.jval.2010.09.011
Rabe, K. F., Martinez, F. J., Ferguson, G. T., Wang, C., Singh, D., Wedzicha, J. A., Trivedi, R., St Rose, E., Ballal, S., McLaren, J., Darken, P., Aurivillius, M., Reisner, C., Dorinsky, P., & ETHOS Investigators. (2020). Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. New England Journal of Medicine, 383(1), 35–48. https://doi.org/10.1056/NEJMoa1916046
Martinez, F. J., Rabe, K. F., Ferguson, G. T., Wedzicha, J. A., Trivedi, R., Jenkins, M., Darken, P., Aurivillius, M., & Dorinsky, P. (2021). Benefits of budesonide/glycopyrrolate/formoterol fumarate (BGF) on symptoms and quality of life in patients with COPD in the ETHOS trial. Respiratory Medicine, 185, 106509. https://doi.org/10.1016/j.rmed.2021.106509
Rabe, K. F., Martinez, F. J., Ferguson, G. T., Wang, C., Singh, D., Wedzicha, J. A., Trivedi, R., St Rose, E., Ballal, S., McLaren, J., Darken, P., Reisner, C., & Dorinsky, P. (2019). A Phase III study of triple therapy with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler 320/18/9.6 μg and 160/18/9.6 μg using co-suspension delivery technology in moderate-to-very severe COPD: The ETHOS study protocol. Respiratory Medicine, 158, 59–66. https://doi.org/10.1016/j.rmed.2019.08.010
Rabe, K. F., Martinez, F. J., Singh, D., Trivedi, R., Jenkins, M., Darken, P., Aurivillius, M., & Dorinsky, P. (2021). Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: A sub-study of the ETHOS trial. Therapeutic Advances in Respiratory Disease, 15, 17534666211034329. https://doi.org/10.1177/17534666211034329
Fairclough, D. L. (2010). Design and analysis of quality of life studies in clinical trials (2nd ed.). Chapman & Hall.
Fenwick, E., Martin, A., Schroeder, M., Mealing, S. J., Solanke, O., Risebrough, N., & Ismaila, A. S. (2021). Cost-effectiveness analysis of a single-inhaler triple therapy for COPD in the UK. ERJ Open Res, 7(1), 00480–02020. https://doi.org/10.1183/23120541.00480-2020
de Nigris, E., Treharne, C., Brighton, N., Holmgren, U., Walker, A., & Haughney, J. (2022). Cost-effectiveness of triple therapy with budesonide/glycopyrronium/formoterol fumarate dihydrate versus dual therapies in moderate-to-very severe chronic obstructive pulmonary disease: United Kingdom analysis using the ETHOS study. International Journal of Chronic Obstructive Pulmonary Disease, 17, 2987–3000. https://doi.org/10.2147/copd.S381138
Kiff, C., Ruiz, S., Varol, N., Gibson, D., Davies, A., & Purkayastha, D. (2018). Cost-effectiveness of roflumilast as an add-on to triple inhaled therapy vs triple inhaled therapy in patients with severe and very severe COPD associated with chronic bronchitis in the UK. International Journal of Chronic Obstructive Pulmonary Disease, 13, 2707–2720. https://doi.org/10.2147/copd.S167730
Martin, A., Shah, D., Ndirangu, K., Anley, G. A., Okorogheye, G., Schroeder, M., Risebrough, N., & Ismaila, A. S. (2022). Is single-inhaler triple therapy for COPD cost-effective in the UK? The IMPACT trial. ERJ Open Res, 8(1), 00333–2021. https://doi.org/10.1183/23120541.00333-2021
Guo, J., Chen, Y., Zhang, W., Tong, S., & Dong, J. (2020). Moderate and severe exacerbations have a significant impact on health-related quality of life, utility, and lung function in patients with chronic obstructive pulmonary disease: A meta-analysis. International Journal of Surgery, 78, 28–35. https://doi.org/10.1016/j.ijsu.2020.04.010
European Network for Health Technology Assessment. (2015). Methods for health economic evaluations—A guideline based on current practices in Europe. Retrieved January 9, 2024, from https://www.eunethta.eu/wp-content/uploads/2018/01/Methods-for-health-economic-evaluations-A-guideline-based-on-current-practices-in-Europe_Guideline_Final-May-2015.pdf.
Bafadhel, M., Rabe, K. F., Martinez, F. J., Singh, D., Darken, P., Jenkins, M., Aurivillius, M., Patel, M., & Dorinsky, P. (2022). Benefits of budesonide/glycopyrronium/formoterol fumarate dihydrate on COPD exacerbations, lung function, symptoms, and quality of life across blood eosinophil ranges: A post-hoc analysis of data from ETHOS. International Journal of Chronic Obstructive Pulmonary Disease, 17, 3061–3073. https://doi.org/10.2147/copd.S374670
Jackson, D., Jenkins, M., de Nigris, E., Patel, M., & Ouwens, M. (2021). The association between health-related quality of life and COPD exacerbations measured by EQ-5D-5L in the ETHOS trial. European Respiratory Journal, 58(suppl 65), PA793. https://doi.org/10.1183/13993003.congress-2021.PA793
DeTora, L. M., Toroser, D., Sykes, A., Vanderlinden, C., Plunkett, F. J., Lane, T., Hanekamp, E., Dormer, L., DiBiasi, F., Bridges, D., Baltzer, L., & Citrome, L. (2022). Good publication practice (GPP) guidelines for company-sponsored biomedical research: 2022 update. Annals of Internal Medicine, 175(9), 1298–1304. https://doi.org/10.7326/M22-1460
Acknowledgments
The authors thank all the patients and their families, and the team of investigators and research staff involved in the ETHOS study. Portions of the data reported in this manuscript have been previously presented at the European Respiratory Society International Congress 2021 [27]. The authors also thank Rona Dawson, James Cranfield, Yvonne Coleman, and Amanda Miller Adams for their review of the plain language summary from the perspective of people living with COPD. Patient reviewers were compensated for their time. Medical writing support, under the guidance of the authors, was provided by Eric Comeau, PhD, and Daniel Spindlow, MSc, CMC Connect, a division of IPG Health Medical Communications, and was funded by AstraZeneca in accordance with Good Publication Practice (GPP 2022) guidelines [28].
Funding
The ETHOS study and the current analyses were supported by AstraZeneca. The study sponsor was involved in the design and conduct of the ETHOS study, in the data analysis and interpretation, and in the review of the manuscript for accuracy. Decisions on final content were made by the authors. Medical writing support for development of this manuscript was funded by AstraZeneca.
Author information
Authors and Affiliations
Contributions
All authors made significant contributions to this work. DJ, EN, and MO were involved in the study conception/design, data analysis, and data interpretation. MJ was involved in the study conception/design, data acquisition, data analysis, and data interpretation. DP was involved in the data acquisition and data analysis. MP was involved in the data interpretation. All authors were involved in drafting, revising, or critically reviewing the report; provided final approval of the version to be published; and have agreed to submission to Quality of Life Research.
Corresponding author
Ethics declarations
Competing interests
Dan Jackson, Martin Jenkins, Mehul Patel, Debasree Purkayastha, and Mario Ouwens are employees of AstraZeneca and hold stock and/or options in the company. Enrico de Negris was an employee of AstraZeneca at the time of analyses.
Ethical approval
The ETHOS study was performed in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with International Council for Harmonisation/Good Clinical Practice, and applicable regulatory requirements. The protocol and informed consent form for the ETHOS study were approved by the appropriate Institutional Review Board, independent ethics committee, or health authority [26].
Consent to participate
Written informed consent was obtained prospectively from all patients before screening.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jackson, D., Jenkins, M., de Nigris, E. et al. Associations between the EQ-5D-5L and exacerbations of chronic obstructive pulmonary disease in the ETHOS trial. Qual Life Res 33, 1029–1039 (2024). https://doi.org/10.1007/s11136-023-03582-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-023-03582-z